Abstract
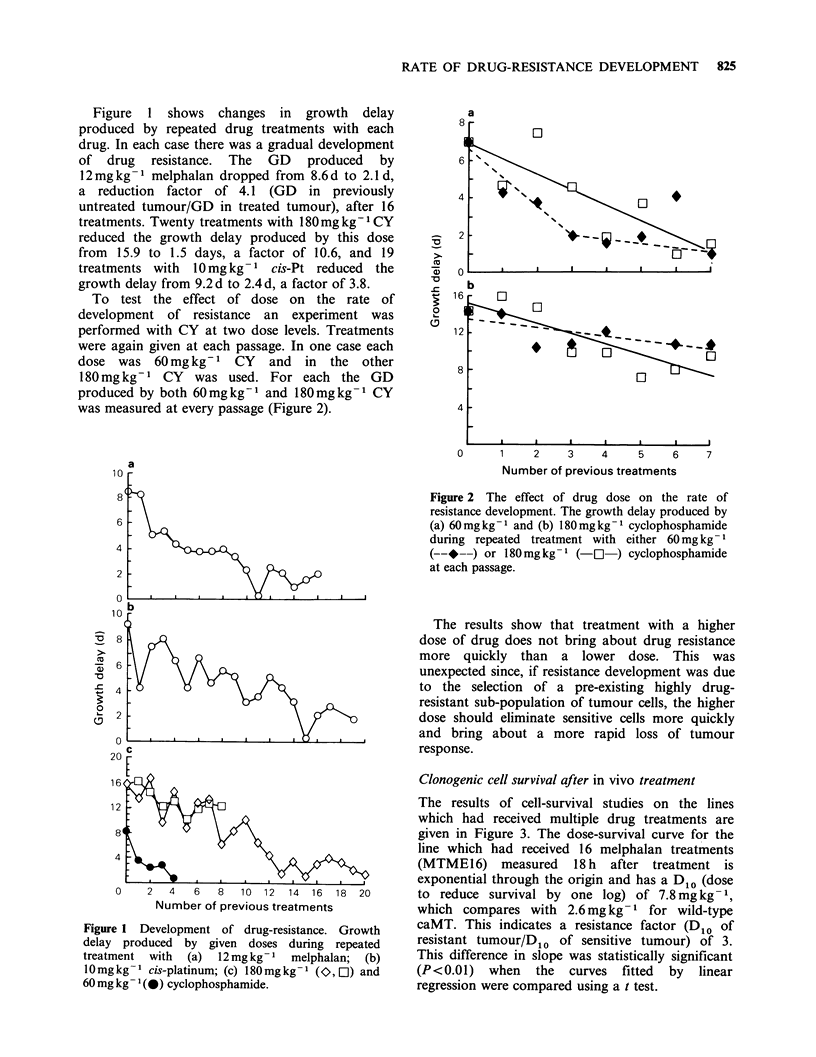
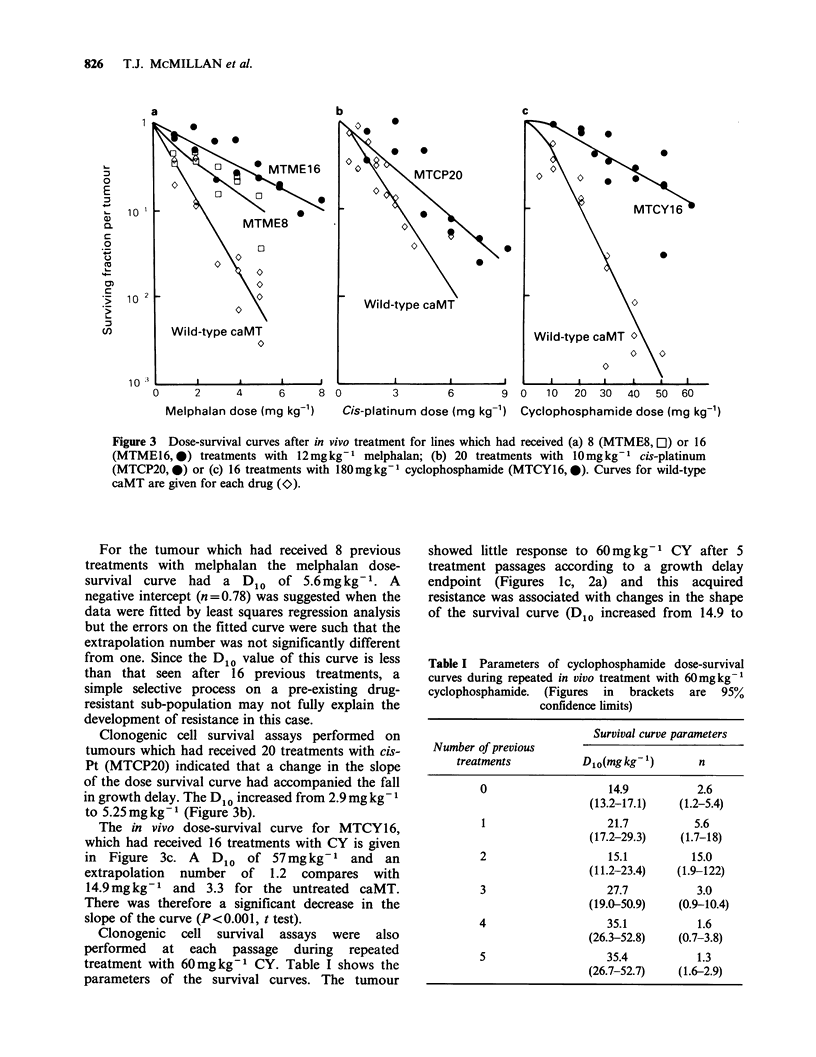
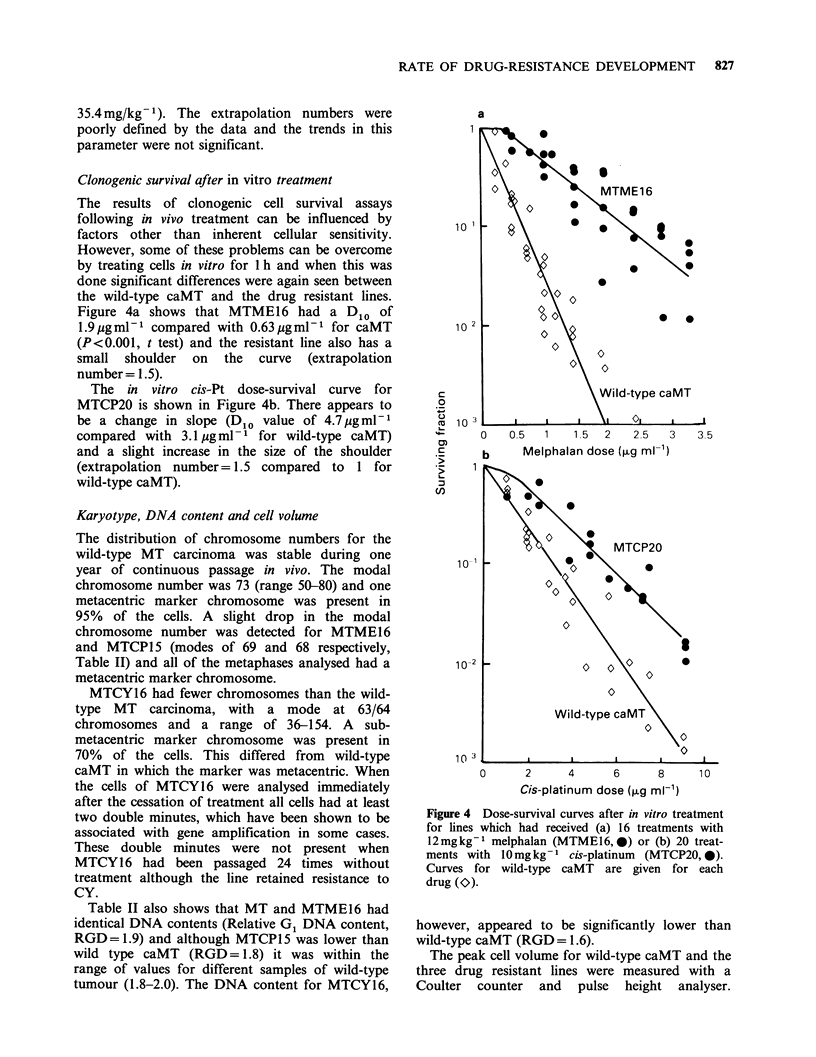
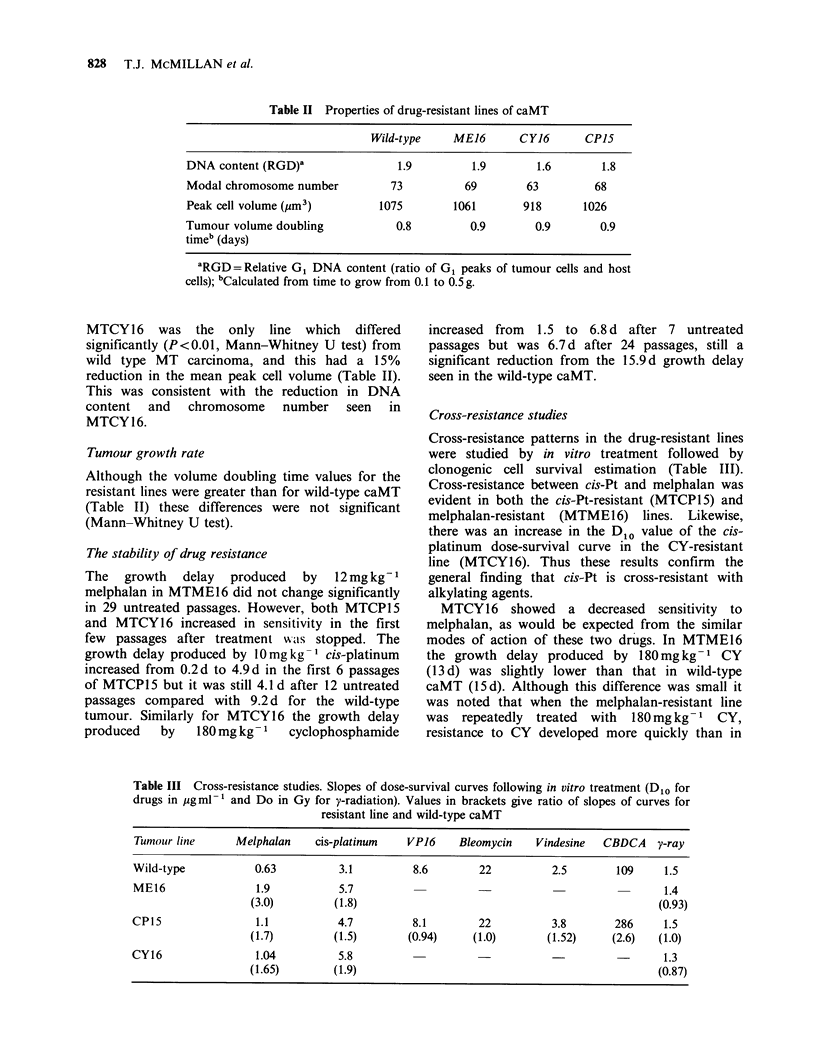
The development of resistance to melphalan, cis-platinum and cyclophosphamide has been examined in the MT murine mammary carcinoma. A gradual decrease in therapeutic response was detected using growth delay and clonogenic cell survival during repeated drug treatment. A slow rate of resistance development, a gradual change in the slope of the dose-survival curves and the inability of 180 mg kg-1 cyclophosphamide to bring about a reduction in tumour response at a faster rate than 60 mg kg-1 cyclophosphamide suggest that resistance development was not due to the selection of a pre-existing highly drug resistant sub-population of tumour cells. Partial drug-resistance is proposed as one possible reason for the apparent inconsistency between these data and existing models of drug-resistance development. The drug-resistant lines were characterized for karyotype, DNA content and cell volume, but only the cyclophosphamide-resistant line showed any significant difference from the wild-type tumour. Cross-resistance studies revealed some inconsistencies with previous reports. Also, resistance to cyclophosphamide developed more quickly in the line which was resistant to melphalan, than in the wild-type tumour, despite the initial appearance of little cross-resistance. This increased rate of resistance development may be important in salvage chemotherapy.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball C. R., Connors T. A., Double J. A., Ujhazy V., Whisson M. E. Comparison of nitrogen-mustard sensitive and -resistant Yoshida sarcomas. Int J Cancer. 1966 Jul 15;1(4):319–327. doi: 10.1002/ijc.2910010403. [DOI] [PubMed] [Google Scholar]
- Bergsagel D. E., Cowan D. H., Hasselback R. Plasma cell myeloma: response of melphalan-resistant patients to high-dose intermittent cyclophosphamide. Can Med Assoc J. 1972 Nov 4;107(9):851–855. [PMC free article] [PubMed] [Google Scholar]
- Berman R., Steel G. G. Induced and inherent resistance to alkylating agents in human small-cell bronchial carcinoma xenografts. Br J Cancer. 1984 Apr;49(4):431–436. doi: 10.1038/bjc.1984.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer M., Smets L. A., Jongsma A. P. Isolation and characterization of subclones of L1210 murine leukemia with different sensitivities to various cytotoxic agents. Cancer Res. 1983 Jun;43(6):2884–2888. [PubMed] [Google Scholar]
- Clements G. B. Selection of biochemically variant, in some cases mutant, mammalian cells in culture. Adv Cancer Res. 1975;21:273–390. doi: 10.1016/s0065-230x(08)60975-6. [DOI] [PubMed] [Google Scholar]
- Courtenay V. D. A soft agar colony assay for Lewis lung tumour and B16 melanoma taken directly from the mouse. Br J Cancer. 1976 Jul;34(1):39–45. doi: 10.1038/bjc.1976.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Incalci M., Torti L., Damia G., Erba E., Morasca L., Garattini S. Ovarian reticular cell sarcoma of the mouse (M5076) made resistant to cyclophosphamide. Cancer Res. 1983 Dec;43(12 Pt 1):5674–5680. [PubMed] [Google Scholar]
- Frondoza C. G., Trivedi S. M., Humphrey R. L. Development and characterization of a cyclophosphamide-resistant mouse plasmacytoma cell line. Cancer Treat Rep. 1982 Jul;66(7):1535–1544. [PubMed] [Google Scholar]
- Goldie J. H., Coldman A. J. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep. 1979 Nov-Dec;63(11-12):1727–1733. [PubMed] [Google Scholar]
- Heppner G. H., Dexter D. L., DeNucci T., Miller F. R., Calabresi P. Heterogeneity in drug sensitivity among tumor cell subpopulations of a single mammary tumor. Cancer Res. 1978 Nov;38(11 Pt 1):3758–3763. [PubMed] [Google Scholar]
- Kaufman R. J., Brown P. C., Schimke R. T. Amplified dihydrofolate reductase genes in unstably methotrexate-resistant cells are associated with double minute chromosomes. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5669–5673. doi: 10.1073/pnas.76.11.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major I. R., Mole R. H. Myeloid leukaemia in x-ray irradiated CBA mice. Nature. 1978 Mar 30;272(5652):455–456. doi: 10.1038/272455a0. [DOI] [PubMed] [Google Scholar]
- Parsons P. G., Morrison L. Melphalan-induced chromosome damage in sensitive and resistant human melanoma cell lines. Int J Cancer. 1978 Apr 15;21(4):438–443. doi: 10.1002/ijc.2910210407. [DOI] [PubMed] [Google Scholar]
- Preisler H. D. Treatment failure in AML. Blood Cells. 1982;8(3):585–602. [PubMed] [Google Scholar]
- Schabel F. M., Jr, Trader M. W., Laster W. R., Jr, Wheeler G. P., Witt M. H. Patterns of resistance and therapeutic synergism among alkylating agents. Antibiot Chemother (1971) 1978;23:200–215. doi: 10.1159/000401484. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Kaufman R. J., Alt F. W., Kellems R. F. Gene amplification and drug resistance in cultured murine cells. Science. 1978 Dec 8;202(4372):1051–1055. doi: 10.1126/science.715457. [DOI] [PubMed] [Google Scholar]
- Schmid F. A., Otter G. M., Stock C. C. Resistance patterns of Walker carcinosarcoma 256 and other rodent tumors to cyclophosphamide and L-phenylalanine mustard. Cancer Res. 1980 Mar;40(3):830–833. [PubMed] [Google Scholar]
- Seeber S., Osieka R., Schmidt C. G., Achterrath W., Crooke S. T. In vivo resistance towards anthracyclines, etoposide, and cis-diamminedichloroplatinum(II). Cancer Res. 1982 Nov;42(11):4719–4725. [PubMed] [Google Scholar]
- Sierocki J. S., Hilaris B. S., Hopfan S., Martini N., Barton D., Golbey R. B., Wittes R. E. cis-Dichlorodiammineplatinum(II) and VP-16-213: an active induction regimen for small cell carcinoma of the lung. Cancer Treat Rep. 1979 Sep-Oct;63(9-10):1593–1597. [PubMed] [Google Scholar]
- Skipper H. E., Schabel F. M., Jr, Lloyd H. H. Experimental therapeutics and kinetics: selection and overgrowth of specifically and permanently drug-resistant tumor cells. Semin Hematol. 1978 Jul;15(3):207–219. [PubMed] [Google Scholar]
- Stephens T. C., Adams K., Peacock J. H. Identification of a subpopulation of MeCCNU resistant cells in previously untreated Lewis lung tumours. Br J Cancer. 1984 Jul;50(1):77–83. doi: 10.1038/bjc.1984.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens T. C., Currie G. A., Peacock J. H. Repopulation of gamma-irradiated Lewis lung carcinoma by malignant cells and host macrophage progenitors. Br J Cancer. 1978 Nov;38(5):573–582. doi: 10.1038/bjc.1978.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens T. C., Peacock J. H. Clonal variation in the sensitivity of B16 melanoma to m-AMSA. Br J Cancer. 1982 Jun;45(6):821–829. doi: 10.1038/bjc.1982.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens T. C., Peacock J. H., Sheldon P. W. Influence of in vitro assay conditions on the assessment of radiobiological parameters of the MT tumour. Br J Radiol. 1980 Dec;53(636):1182–1187. doi: 10.1259/0007-1285-53-636-1182. [DOI] [PubMed] [Google Scholar]
- Tew K. D., Moy B. C., Hartley-Asp B. Acquired drug resistance is accompanied by modification in the karyotype and nuclear matrix of a rat carcinoma cell line. Exp Cell Res. 1983 Dec;149(2):443–450. doi: 10.1016/0014-4827(83)90356-7. [DOI] [PubMed] [Google Scholar]


