Abstract
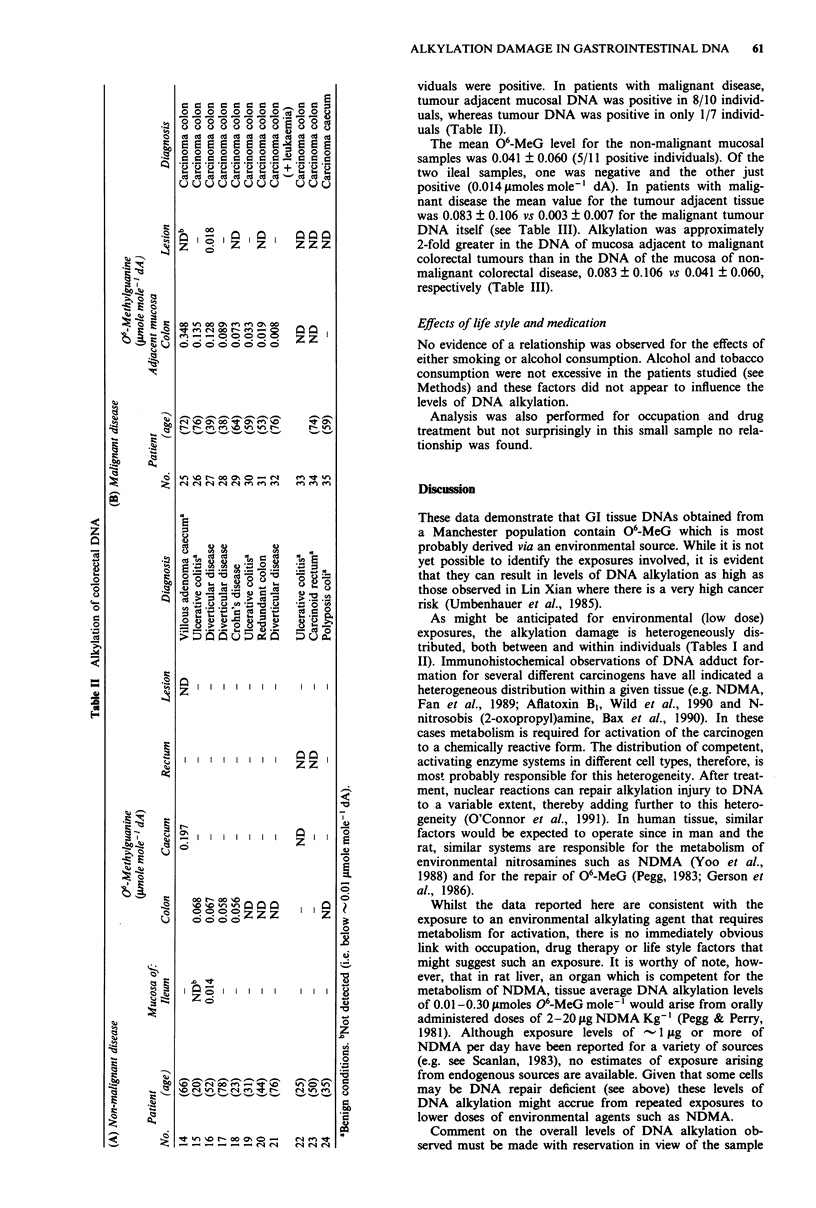
Damage arising from putative environmental sources has been found in the DNA of the gastric and colorectal mucosae of patients presenting with gastrointestinal disorders from the South Manchester area. O6-Methylguanine (O6-MeG) in the range 0.010- greater than 0.300 mu moles mole-1 adenine was heterogeneously distributed both between and within individuals. The pattern of alkylation of tissue DNA appears to differ when comparison is made between gastric and colorectal samples. Most of the gastric tumour DNA samples were alkylated (5/6; 0.087 +/- 0.097), whereas the DNA of the associated mucosa was alkylated less frequently (2/7) and to a lesser extent; (0.017 +/- 0.030; P = 0.07). Conversely, colorectal tumour DNA was alkylated infrequently (1/7) and to a lower extent (0.003 +/- 0.007) than the DNA of the adjacent mucosa (8/10 samples alkylated with a mean of 0.083 +/- 0.106; P = less than 0.01), or indeed of any other tissue. Although increased levels of DNA damage in tissue associated with malignant disease have been indicated by independent studies of DNA damage at other cancer sites, significant differences were not observed in the present report, neither was there any suggestion of a relationship with smoking or alcohol consumption. The data provided by this report indicate that exposure to putative environmental alkylating agents occurs in the UK at levels comparable to those previously detected in areas of higher cancer risk. Although we cannot determine the extent to which this DNA damage is attributable to normal background exposures, it is evident that the alkylation of tissue DNA occurs and is not uniform. In conjunction with other reports, therefore these differences may begin to provide indications of mechanisms that could be of relevance in the aetiology of gastrointestinal cancers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bos J. L. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep 1;49(17):4682–4689. [PubMed] [Google Scholar]
- Boyle J. M., Durrant L. G., Wild C. P., Saffhill R., Margison G. P. Genetic evidence for nucleotide excision repair of O6-alkylguanine in mammalian cells. J Cell Sci Suppl. 1987;6:147–160. doi: 10.1242/jcs.1984.supplement_6.10. [DOI] [PubMed] [Google Scholar]
- Boyle J. M., Saffhill R., Margison G. P., Fox M. A comparison of cell survival, mutation and persistence of putative promutagenic lesions in Chinese hamster cells exposed to BNU or MNU. Carcinogenesis. 1986 Dec;7(12):1981–1985. doi: 10.1093/carcin/7.12.1981. [DOI] [PubMed] [Google Scholar]
- Cameron R., Sweeney G. D., Jones K., Lee G., Farber E. A relative deficiency of cytochrome P-450 and aryl hydrocarbon [benzo(a)pyrene] hydroxylase in hyperplastic nodules induced by 2-acetylaminofluorene in rat liver. Cancer Res. 1976 Nov;36(11 Pt 1):3888–3893. [PubMed] [Google Scholar]
- Cuzick J., Routledge M. N., Jenkins D., Garner R. C. DNA adducts in different tissues of smokers and non-smokers. Int J Cancer. 1990 Apr 15;45(4):673–678. doi: 10.1002/ijc.2910450417. [DOI] [PubMed] [Google Scholar]
- Doll R. Epidemiology and the prevention of cancer: some recent developments. J Cancer Res Clin Oncol. 1988;114(5):447–458. doi: 10.1007/BF00391491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C. Y., Butler W. H., O'Connor P. J. Cell and tissue specific localization of O6-methylguanine in the DNA of rats given N-nitrosodimethylamine: effects of protein deficient and normal diets. Carcinogenesis. 1989 Oct;10(10):1967–1970. doi: 10.1093/carcin/10.10.1967. [DOI] [PubMed] [Google Scholar]
- Farber E., Parker S., Gruenstein M. The resistance of putative premalignant liver cell populations, hyperplastic nodules, to the acute cytotoxic effects of some hepatocarcinogens. Cancer Res. 1976 Nov;36(11 Pt 1):3879–3887. [PubMed] [Google Scholar]
- Foiles P. G., Miglietta L. M., Akerkar S. A., Everson R. B., Hecht S. S. Detection of O6-methyldeoxyguanosine in human placental DNA. Cancer Res. 1988 Aug 1;48(15):4184–4188. [PubMed] [Google Scholar]
- Forman D. Are nitrates a significant risk factor in human cancer? Cancer Surv. 1989;8(2):443–458. [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. The role of adenosine and 2'-deoxyadenosine in mammalian cells. Annu Rev Biochem. 1978;47:655–686. doi: 10.1146/annurev.bi.47.070178.003255. [DOI] [PubMed] [Google Scholar]
- Gerson S. L., Trey J. E., Miller K., Berger N. A. Comparison of O6-alkylguanine-DNA alkyltransferase activity based on cellular DNA content in human, rat and mouse tissues. Carcinogenesis. 1986 May;7(5):745–749. doi: 10.1093/carcin/7.5.745. [DOI] [PubMed] [Google Scholar]
- Huh N. H., Satoh M. S., Shiga J., Rajewsky M. F., Kuroki T. Immunoanalytical detection of O4-ethylthymine in liver DNA of individuals with or without malignant tumors. Cancer Res. 1989 Jan 1;49(1):93–97. [PubMed] [Google Scholar]
- Kyrtopoulos S. A., Ampatzi P., Davaris P., Haritopoulos N., Golematis B. Studies in gastric carcinogenesis. IV. O6-methylguanine and its repair in normal and atrophic biopsy specimens of human gastric mucosa. Correlation of O6-alkylguanine-DNA alkyltransferase activities in gastric mucosa and circulating lymphocytes. Carcinogenesis. 1990 Mar;11(3):431–436. doi: 10.1093/carcin/11.3.431. [DOI] [PubMed] [Google Scholar]
- Licht W. R., Deen W. M. Theoretical model for predicting rates of nitrosamine and nitrosamide formation in the human stomach. Carcinogenesis. 1988 Dec;9(12):2227–2237. doi: 10.1093/carcin/9.12.2227. [DOI] [PubMed] [Google Scholar]
- Lu S. H., Ohshima H., Fu H. M., Tian Y., Li F. M., Blettner M., Wahrendorf J., Bartsch H. Urinary excretion of N-nitrosamino acids and nitrate by inhabitants of high- and low-risk areas for esophageal cancer in Northern China: endogenous formation of nitrosoproline and its inhibition by vitamin C. Cancer Res. 1986 Mar;46(3):1485–1491. [PubMed] [Google Scholar]
- Magee P. N. The experimental basis for the role of nitroso compounds in human cancer. Cancer Surv. 1989;8(2):207–239. [PubMed] [Google Scholar]
- Myers K. A., Saffhill R., O'Connor P. J. Repair of alkylated purines in the hepatic DNA of mitochondria and nuclei in the rat. Carcinogenesis. 1988 Feb;9(2):285–292. doi: 10.1093/carcin/9.2.285. [DOI] [PubMed] [Google Scholar]
- O'Connor P. J., Saffhill R. The action of rat cytosol enzymes on some methylated nucleic acid components produced by the carcinogenic N-nitroso compounds. Chem Biol Interact. 1979 Jun;26(1):91–102. doi: 10.1016/0009-2797(79)90095-4. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Perry W. Alkylation of nucleic acids and metabolism of small doses of dimethylnitrosamine in the rat. Cancer Res. 1981 Aug;41(8):3128–3132. [PubMed] [Google Scholar]
- Phillips D. H., Hewer A., Martin C. N., Garner R. C., King M. M. Correlation of DNA adduct levels in human lung with cigarette smoking. Nature. 1988 Dec 22;336(6201):790–792. doi: 10.1038/336790a0. [DOI] [PubMed] [Google Scholar]
- Preston-Martin S., Correa P. Epidemiological evidence for the role of nitroso compounds in human cancer. Cancer Surv. 1989;8(2):459–473. [PubMed] [Google Scholar]
- Saffhill R., Fida S., Bromley M., O'Connor P. J. Promutagenic alkyl lesions are induced in the tissue DNA of animals treated with isoniazid. Hum Toxicol. 1988 Jul;7(4):311–317. doi: 10.1177/096032718800700403. [DOI] [PubMed] [Google Scholar]
- Saffhill R., Margison G. P., O'Connor P. J. Mechanisms of carcinogenesis induced by alkylating agents. Biochim Biophys Acta. 1985 Dec 17;823(2):111–145. doi: 10.1016/0304-419x(85)90009-5. [DOI] [PubMed] [Google Scholar]
- Swenberg J. A., Dyroff M. C., Bedell M. A., Popp J. A., Huh N., Kirstein U., Rajewsky M. F. O4-ethyldeoxythymidine, but not O6-ethyldeoxyguanosine, accumulates in hepatocyte DNA of rats exposed continuously to diethylnitrosamine. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1692–1695. doi: 10.1073/pnas.81.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbenhauer D., Wild C. P., Montesano R., Saffhill R., Boyle J. M., Huh N., Kirstein U., Thomale J., Rajewsky M. F., Lu S. H. O(6)-methyldeoxyguanosine in oesophageal DNA among individuals at high risk of oesophageal cancer. Int J Cancer. 1985 Dec 15;36(6):661–665. doi: 10.1002/ijc.2910360607. [DOI] [PubMed] [Google Scholar]
- Wiestler O., Kleihues P., Pegg A. E. O6-alkylguanine-DNA alkyltransferase activity in human brain and brain tumors. Carcinogenesis. 1984 Jan;5(1):121–124. doi: 10.1093/carcin/5.1.121. [DOI] [PubMed] [Google Scholar]
- Wild C. P., Montesano R., Van Benthem J., Scherer E., Den Engelse L. Intercellular variation in levels of adducts of aflatoxin B1 and G1 in DNA from rat tissues: a quantitative immunocytochemical study. J Cancer Res Clin Oncol. 1990;116(2):134–140. doi: 10.1007/BF01612667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild C. P., Smart G., Saffhill R., Boyle J. M. Radioimmunoassay of O6-methyldeoxyguanosine in DNA of cells alkylated in vitro and in vivo. Carcinogenesis. 1983 Dec;4(12):1605–1609. doi: 10.1093/carcin/4.12.1605. [DOI] [PubMed] [Google Scholar]
- Wilson V. L., Weston A., Manchester D. K., Trivers G. E., Roberts D. W., Kadlubar F. F., Wild C. P., Montesano R., Willey J. C., Mann D. L. Alkyl and aryl carcinogen adducts detected in human peripheral lung. Carcinogenesis. 1989 Nov;10(11):2149–2153. doi: 10.1093/carcin/10.11.2149. [DOI] [PubMed] [Google Scholar]
- Yarosh D. B. The role of O6-methylguanine-DNA methyltransferase in cell survival, mutagenesis and carcinogenesis. Mutat Res. 1985 Jan-Mar;145(1-2):1–16. doi: 10.1016/0167-8817(85)90034-3. [DOI] [PubMed] [Google Scholar]
- Yoo J. S., Guengerich F. P., Yang C. S. Metabolism of N-nitrosodialkylamines by human liver microsomes. Cancer Res. 1988 Mar 15;48(6):1499–1504. [PubMed] [Google Scholar]



