Abstract
1-beta-D-Arabinofuranosylcytosine (Ara-C) at a concentration which inhibits nuclear-DNA reduplication (0.05 microM), enhances mitochondrial activities like respiration, in cell of a human leukaemic cell line Molt 4. While the specific activity of cytochrome c oxidase doubles in the course of the G1 phase of the cell cycle in control cells, in the presence of Ara-C G1 phase cells begin to increase the enzyme activity earlier and show a 3-fold rise of the enzyme activity in the same period of time. This is explained by an enhanced expression of the mitochondrial genome: the concentration of transcripts for the mitochondrially encoded subunit II of cytochrome c oxidase increases. Inhibition of mitochondrial protein synthesis abolishes the Ara-C induced effect on the specific activity of cytochrome c oxidase activity. The concentration of transcripts of the nuclearly encoded subunit IV of cytochrome c oxidase is the same as in control cells, and also the specific activity of the mitochondrial enzyme citrate synthase, which is exclusively encoded on nuclear-DNA, is not affected by Ara-C. Dysregulation in time and intensity of the expression of the mitochondrial relative to the nuclear genome may impair cell function and reflect a till now neglected mechanism of Ara-C cytotoxicity.
Full text
PDF

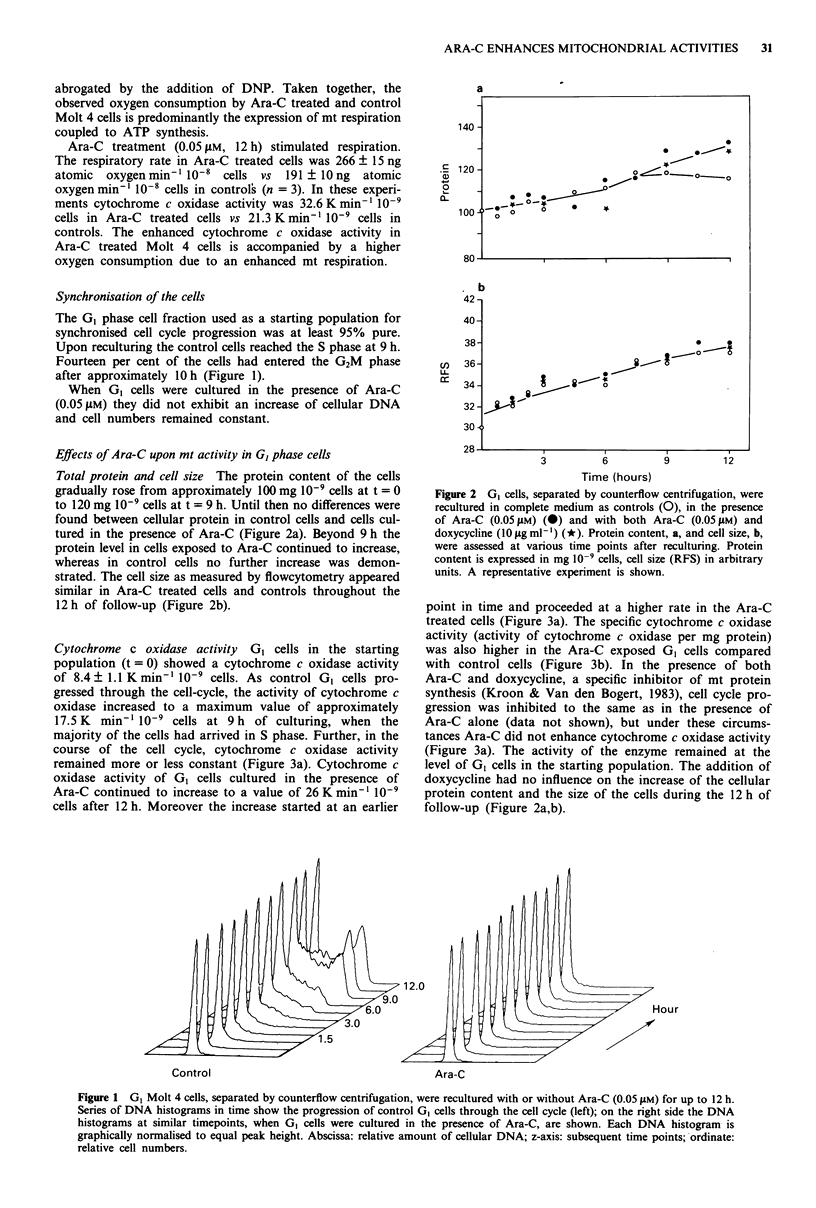
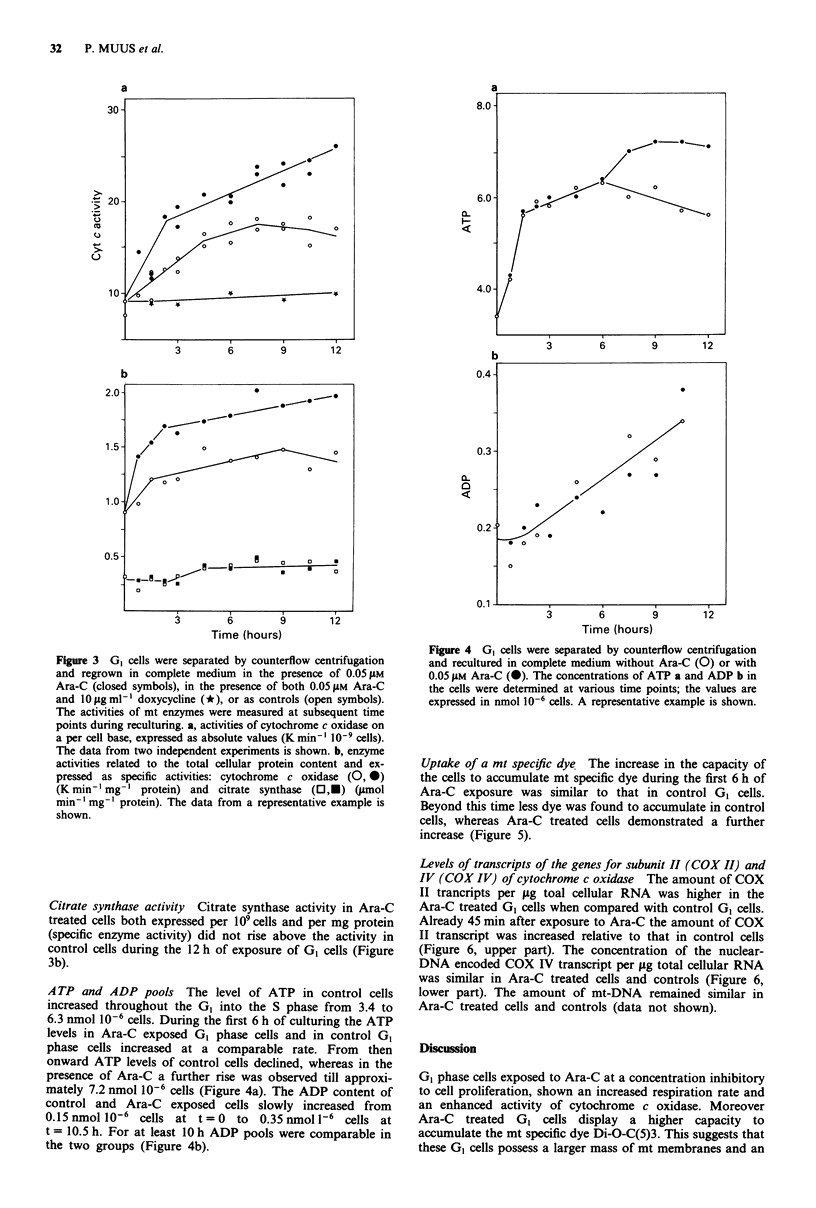
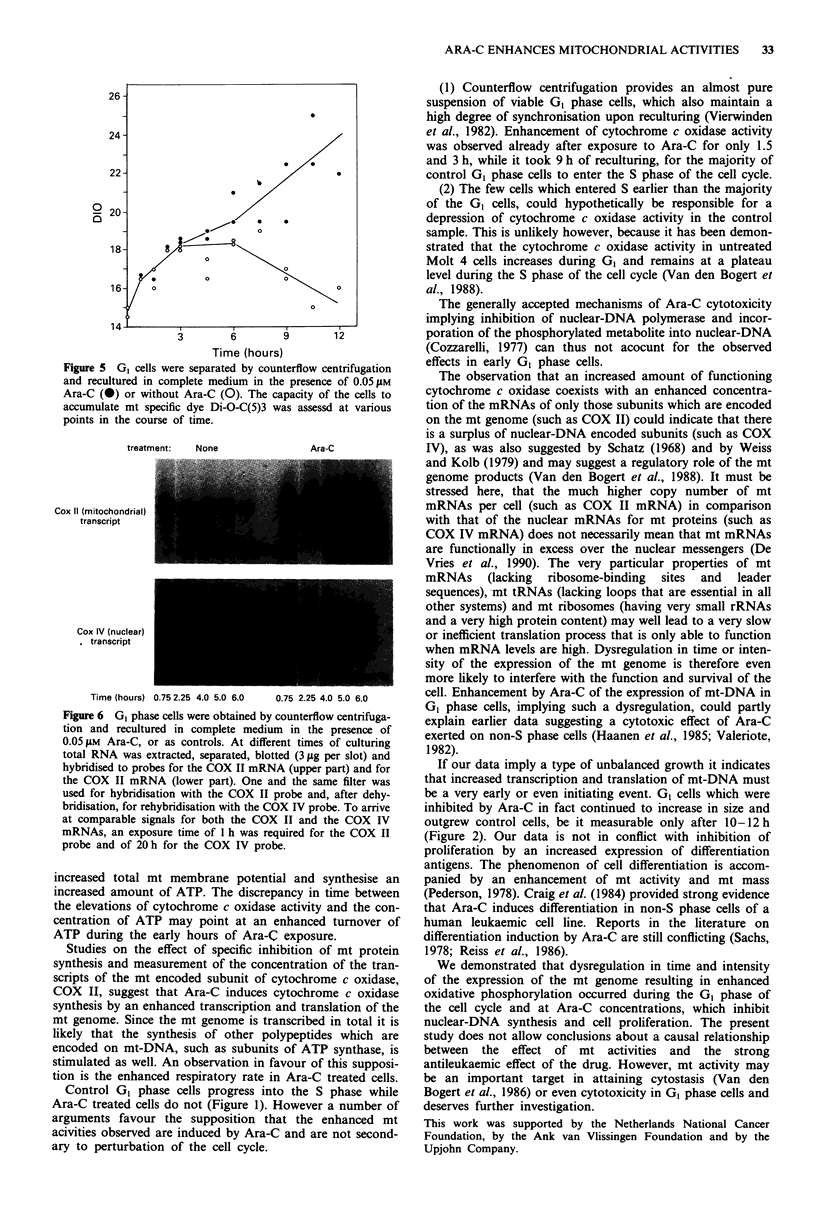

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C. Rapid extraction of high molecular weight RNA from cultured cells and granulocytes for Northern analysis. Nucleic Acids Res. 1988 Feb 25;16(4):1487–1497. doi: 10.1093/nar/16.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Ruttenberg J. C., Kroon A. M. Mitochondrial DNA. I. Preparation and properties of mitochondrial DNA from chick liver. Biochim Biophys Acta. 1967 Nov 21;149(1):140–155. doi: 10.1016/0005-2787(67)90697-1. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R. The mechanism of action of inhibitors of DNA synthesis. Annu Rev Biochem. 1977;46:641–668. doi: 10.1146/annurev.bi.46.070177.003233. [DOI] [PubMed] [Google Scholar]
- Craig R. W., Frankfurt O. S., Sakagami H., Takeda K., Bloch A. Macromolecular and cell cycle effects of different classes of agents inducing the maturation of human myeloblastic leukemia (ML-1) cells. Cancer Res. 1984 Jun;44(6):2421–2429. [PubMed] [Google Scholar]
- Denhardt D. T., Edwards D. R., Parfett C. L. Gene expression during the mammalian cell cycle. Biochim Biophys Acta. 1986 Oct 28;865(2):83–125. doi: 10.1016/0304-419x(86)90024-7. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Lehninger A. L. Sites of inhibition of mitochondrial electron transport in macrophage-injured neoplastic cells. J Cell Biol. 1982 Nov;95(2 Pt 1):527–535. doi: 10.1083/jcb.95.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haanen C., Muus P., Pennings A. The effect of cytosine arabinoside upon mitochondrial staining kinetics in human hematopoietic cells. Histochemistry. 1986;84(4-6):609–613. doi: 10.1007/BF00482999. [DOI] [PubMed] [Google Scholar]
- Haanen C., Muus P., Raymakers R., Drenthe-Schonk A., Salden M., Wessels J. Studies on the cytotoxicity of cytosine arabinoside. Semin Oncol. 1985 Jun;12(2 Suppl 3):120–129. [PubMed] [Google Scholar]
- James T. W., Bohman R. Proliferation of mitochondria during the cell cycle of the human cell line (HL-60). J Cell Biol. 1981 May;89(2):256–260. doi: 10.1083/jcb.89.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Bockus B. J., Chen L. B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J Cell Biol. 1981 Mar;88(3):526–535. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975 Jul;66(1):188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon A. M., Van den Bogert C. Antibacterial drugs and their interference with the biogenesis of mitochondria in animal and human cells. Pharm Weekbl Sci. 1983 Jun 24;5(3):81–87. doi: 10.1007/BF01960982. [DOI] [PubMed] [Google Scholar]
- Kufe D. W., Major P. P., Egan E. M., Beardsley G. P. Correlation of cytotoxicity with incorporation of ara-C into DNA. J Biol Chem. 1980 Oct 10;255(19):8997–8900. [PubMed] [Google Scholar]
- Mariottini P., Chomyn A., Riley M., Cottrell B., Doolittle R. F., Attardi G. Identification of the polypeptides encoded in the unassigned reading frames 2, 4, 4L, and 5 of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1563–1567. doi: 10.1073/pnas.83.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muus P., Haanen C., Pennings A., Ruitenbeek W., Van den Bogert C. Influence of cytarabine on mitochondrial function and mitochondrial biogenesis. Semin Oncol. 1987 Jun;14(2 Suppl 1):245–250. [PubMed] [Google Scholar]
- Pedersen P. L. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Plas A., de Witte T., Wessels H., Haanen C. A new multichamber counterflow centrifugation rotor with high-separation capacity and versatile potentials. Exp Hematol. 1988 Jun;16(5):355–359. [PubMed] [Google Scholar]
- Reiss M., Gamba-Vitalo C., Sartorelli A. C. Induction of tumor cell differentiation as a therapeutic approach: preclinical models for hematopoietic and solid neoplasms. Cancer Treat Rep. 1986 Jan;70(1):201–218. [PubMed] [Google Scholar]
- Schatz G., Butow R. A. How are proteins imported into mitochondria? Cell. 1983 Feb;32(2):316–318. doi: 10.1016/0092-8674(83)90450-6. [DOI] [PubMed] [Google Scholar]
- Schatz G. Impaired binding of mitochondrial adenosine triphosphatase in the cytoplasmic "petite" mutant of Saccharomyces cerevisiae. J Biol Chem. 1968 May 10;243(9):2192–2199. [PubMed] [Google Scholar]
- Van den Bogert C., Muus P., Haanen C., Pennings A., Melis T. E., Kroon A. M. Mitochondrial biogenesis and mitochondrial activity during the progression of the cell cycle of human leukemic cells. Exp Cell Res. 1988 Sep;178(1):143–153. doi: 10.1016/0014-4827(88)90385-0. [DOI] [PubMed] [Google Scholar]
- Vierwinden G., Drenthe-Schonk A. M., Plas A. M., Linssen P. C., Pennings A. H., Holdrinet R. S., van Egmond J., Wessels J. M., Haanen C. A. Variations of the phosphorylation of 1-beta-D-arabinofuranosylcytosine (ARA-C) in human myeloid leukemic cells related to the cell cycle. Leuk Res. 1982;6(2):251–259. doi: 10.1016/0145-2126(82)90031-5. [DOI] [PubMed] [Google Scholar]
- Weiss H., Kolb H. J. Isolation of mitochondrial succinate: ubiquinone reductase, cytochrome c reductase and cytochrome c oxidase from Neurospora crassa using nonionic detergent. Eur J Biochem. 1979 Aug 15;99(1):139–149. doi: 10.1111/j.1432-1033.1979.tb13240.x. [DOI] [PubMed] [Google Scholar]
- Woodcock D. M. Cytosine arabinoside toxicity: molecular events, biological consequences, and their implications. Semin Oncol. 1987 Jun;14(2 Suppl 1):251–256. [PubMed] [Google Scholar]
- de Vries H., van 't Sant P. Interplay between different genetic systems in eukaryotic cells: nucleocytoplasmic-mitochondrial interrelations. Horiz Biochem Biophys. 1983;7:249–277. [PubMed] [Google Scholar]
- de Witte T., Plas A., Koekman E., Blankenborg G., Salden M., Wessels J., Haanen C. Separation of human bone marrow by counterflow centrifugation monitored by DNA-flowcytometry. Br J Haematol. 1984 Oct;58(2):249–258. doi: 10.1111/j.1365-2141.1984.tb06083.x. [DOI] [PubMed] [Google Scholar]
- van den Bogert C., van Kernebeek G., de Leij L., Kroon A. M. Inhibition of mitochondrial protein synthesis leads to proliferation arrest in the G1-phase of the cell cycle. Cancer Lett. 1986 Jul;32(1):41–51. doi: 10.1016/0304-3835(86)90037-6. [DOI] [PubMed] [Google Scholar]