Abstract
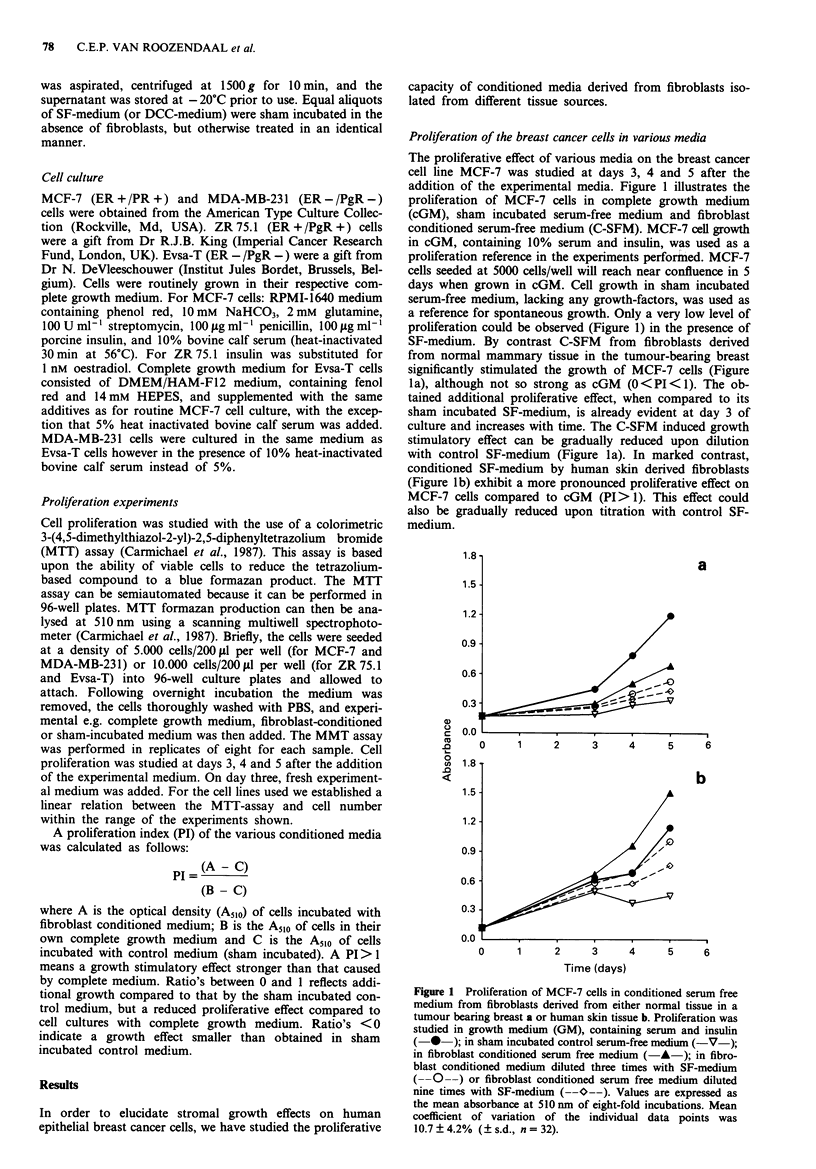
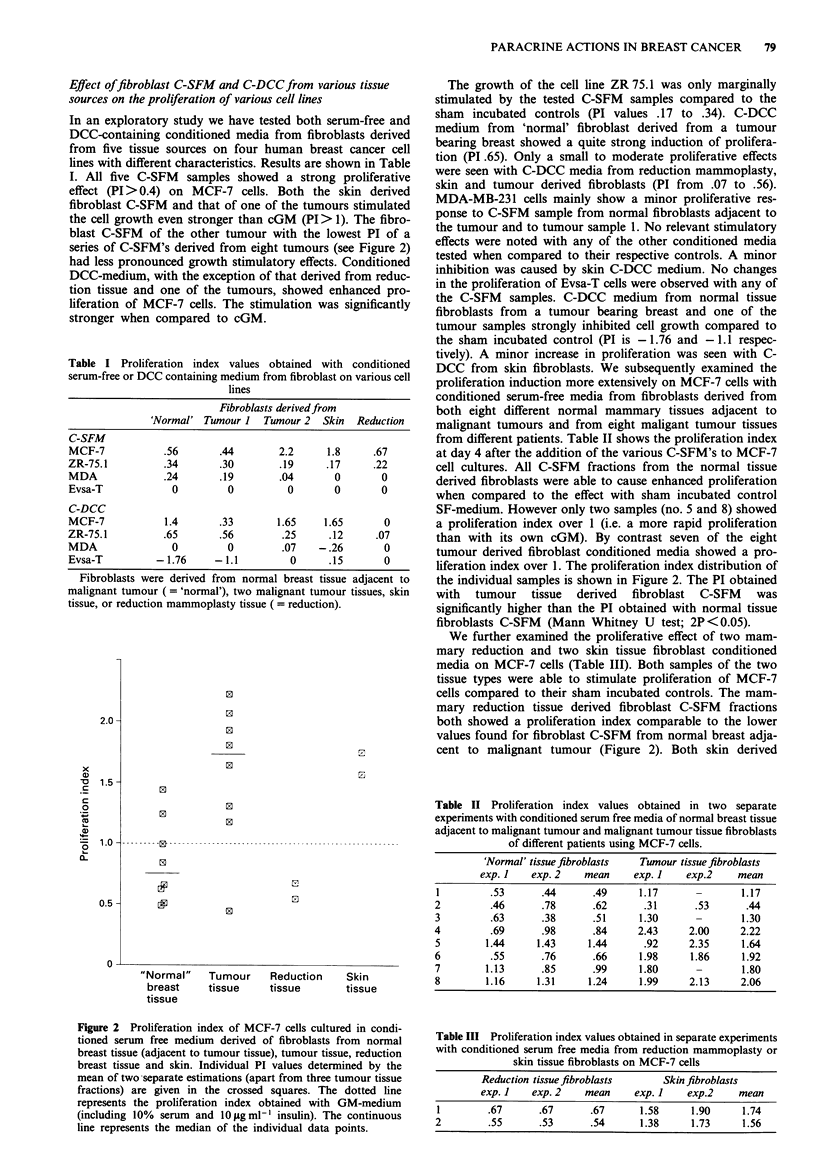
Paracrine influences from fibroblasts derived from different sources of breast tissue on epithelial breast cancer cell growth in vitro were investigated. Medium conditioned (CM) by fibroblasts derived from tumours, adjacent normal breast tissue, and normal breast tissue obtained from reduction mammoplasty or from skin tissue significantly stimulated the growth of the steroid-receptor positive cell lines MCF-7 and ZR 75.1. The proliferation index (PI) on MCF-7 cells with CM from fibroblasts derived from breast tumour tissue was significantly higher than that obtained with fibroblasts derived from adjacent normal breast tissue (2p less than 0.05, n = 8). The PI obtained with CM from normal fibroblast cultures from reduction mammoplasty tissue, like normal tissue adjacent to the tumour, fell in the lower range of values. Skin fibroblast, like tumour tissue derived fibroblast, CM caused a high range PI. MDA-MB-231 and Evsa-T, two steroid-receptor negative cell lines, showed only a minor growth stimulatory responses with some of the fibroblast CM's. Evsa-T was occasionally inhibited by CM's. In conclusion, stromal factors play a role in the growth regulation of human breast cancer cells. The effects on cancer cell growth are, however, varying depending on the source of the stroma and the characteristics of the epithelial tumour cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. F., Newton C. J., Braunsberg H., Shaikh N., Ghilchik M., James V. H. Effects of human breast fibroblasts on growth and 17 beta-estradiol dehydrogenase activity of MCF-7 cells in culture. Breast Cancer Res Treat. 1988 May;11(2):165–172. doi: 10.1007/BF01805840. [DOI] [PubMed] [Google Scholar]
- Adams E. F., Newton C. J., Tait G. H., Braunsberg H., Reed M. J., James V. H. Paracrine influence of human breast stromal fibroblasts on breast epithelial cells: secretion of a polypeptide which stimulates reductive 17 beta-oestradiol dehydrogenase activity. Int J Cancer. 1988 Jul 15;42(1):119–122. doi: 10.1002/ijc.2910420122. [DOI] [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Clemmons D. R. Multiple hormones stimulate the production of somatomedin by cultured human fibroblasts. J Clin Endocrinol Metab. 1984 May;58(5):850–856. doi: 10.1210/jcem-58-5-850. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Underwood L. E., Van Wyk J. J. Hormonal control of immunoreactive somatomedin production by cultured human fibroblasts. J Clin Invest. 1981 Jan;67(1):10–19. doi: 10.1172/JCI110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colletta A. A., Wakefield L. M., Howell F. V., van Roozendaal K. E., Danielpour D., Ebbs S. R., Sporn M. B., Baum M. Anti-oestrogens induce the secretion of active transforming growth factor beta from human fetal fibroblasts. Br J Cancer. 1990 Sep;62(3):405–409. doi: 10.1038/bjc.1990.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. B., Kasid A., Huff K. K., Bates S. E., Knabbe C., Bronzert D., Gelmann E. P., Lippman M. E. Activation of growth factor secretion in tumorigenic states of breast cancer induced by 17 beta-estradiol or v-Ha-ras oncogene. Proc Natl Acad Sci U S A. 1987 Feb;84(3):837–841. doi: 10.1073/pnas.84.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enami J., Enami S., Koga M. Growth of normal and neoplastic mouse mammary epithelial cells in primary culture: stimulation by conditioned medium from mouse mammary fibroblasts. Gan. 1983 Dec;74(6):845–853. [PubMed] [Google Scholar]
- Ervin P. R., Jr, Kaminski M. S., Cody R. L., Wicha M. S. Production of mammastatin, a tissue-specific growth inhibitor, by normal human mammary cells. Science. 1989 Jun 30;244(4912):1585–1587. doi: 10.1126/science.2662405. [DOI] [PubMed] [Google Scholar]
- Garin-Chesa P., Old L. J., Rettig W. J. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam S. Z. Mammary fibroblast influence on normal mouse mammary epithelial cell responses to estrogen in vitro. Cancer Res. 1986 Jan;46(1):310–316. [PubMed] [Google Scholar]
- Horgan K., Jones D. L., Mansel R. E. Mitogenicity of human fibroblasts in vivo for human breast cancer cells. Br J Surg. 1987 Mar;74(3):227–229. doi: 10.1002/bjs.1800740326. [DOI] [PubMed] [Google Scholar]
- Lippman M. E., Dickson R. B., Kasid A., Gelmann E., Davidson N., McManaway M., Huff K., Bronzert D., Bates S., Swain S. Autocrine and paracrine growth regulation of human breast cancer. J Steroid Biochem. 1986 Jan;24(1):147–154. doi: 10.1016/0022-4731(86)90044-0. [DOI] [PubMed] [Google Scholar]
- McGrath C. M. Augmentation of the response of normal mammary epithelial cells to estradiol by mammary stroma. Cancer Res. 1983 Mar;43(3):1355–1360. [PubMed] [Google Scholar]
- Miller F. R., McInerney D. Epithelial component of host-tumor interactions in the orthotopic site preference of a mouse mammary tumor. Cancer Res. 1988 Jul 1;48(13):3698–3701. [PubMed] [Google Scholar]
- Osborne C. K., Arteaga C. L. Autocrine and paracrine growth regulation of breast cancer: clinical implications. Breast Cancer Res Treat. 1990 Jan;15(1):3–11. doi: 10.1007/BF01811884. [DOI] [PubMed] [Google Scholar]
- Osborne C. K., Clemmons D. R., Arteaga C. L. Regulation of breast cancer growth by insulin-like growth factors. J Steroid Biochem Mol Biol. 1990 Dec 20;37(6):805–809. doi: 10.1016/0960-0760(90)90423-i. [DOI] [PubMed] [Google Scholar]
- Pekonen F., Partanen S., Mäkinen T., Rutanen E. M. Receptors for epidermal growth factor and insulin-like growth factor I and their relation to steroid receptors in human breast cancer. Cancer Res. 1988 Mar 1;48(5):1343–1347. [PubMed] [Google Scholar]
- Peyrat J. P., Bonneterre J., Laurent J. C., Louchez M. M., Amrani S., Leroy-Martin B., Vilain M. O., Delobelle A., Demaille A. Presence and characterization of insulin-like growth factor 1 receptors in human benign breast disease. Eur J Cancer Clin Oncol. 1988 Sep;24(9):1425–1431. doi: 10.1016/0277-5379(88)90332-x. [DOI] [PubMed] [Google Scholar]
- Rosen N., Yee D., Lippman M. E., Paik S., Cullen K. J. Insulin-like growth factors in human breast cancer. Breast Cancer Res Treat. 1991 May;18 (Suppl 1):S55–S62. doi: 10.1007/BF02633529. [DOI] [PubMed] [Google Scholar]
- Story M. T., Livingston B., Baeten L., Swartz S. J., Jacobs S. C., Begun F. P., Lawson R. K. Cultured human prostate-derived fibroblasts produce a factor that stimulates their growth with properties indistinguishable from basic fibroblast growth factor. Prostate. 1989;15(4):355–365. doi: 10.1002/pros.2990150408. [DOI] [PubMed] [Google Scholar]
- Yee D., Cullen K. J., Paik S., Perdue J. F., Hampton B., Schwartz A., Lippman M. E., Rosen N. Insulin-like growth factor II mRNA expression in human breast cancer. Cancer Res. 1988 Dec 1;48(23):6691–6696. [PubMed] [Google Scholar]
- Yee D., Favoni R. E., Lippman M. E., Powell D. R. Identification of insulin-like growth factor binding proteins in breast cancer cells. Breast Cancer Res Treat. 1991 Mar;18(1):3–10. doi: 10.1007/BF01975437. [DOI] [PubMed] [Google Scholar]



