Abstract
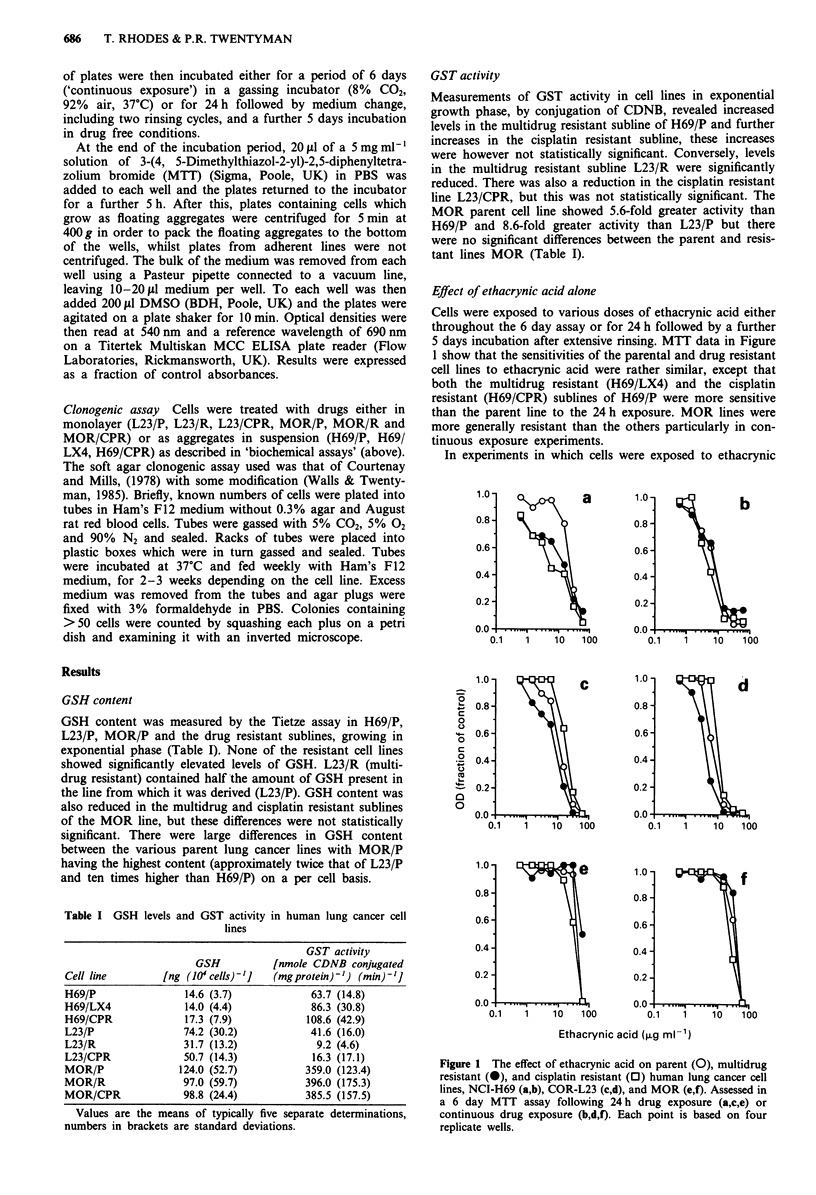
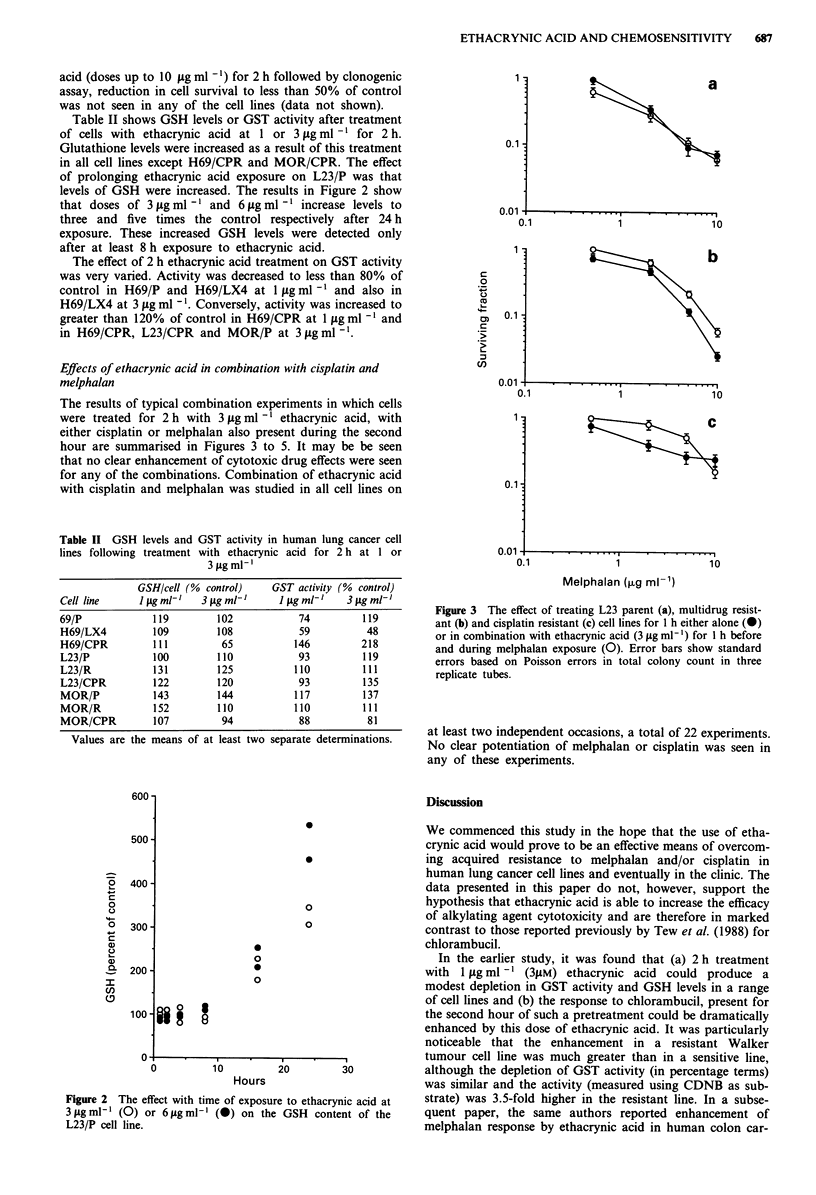
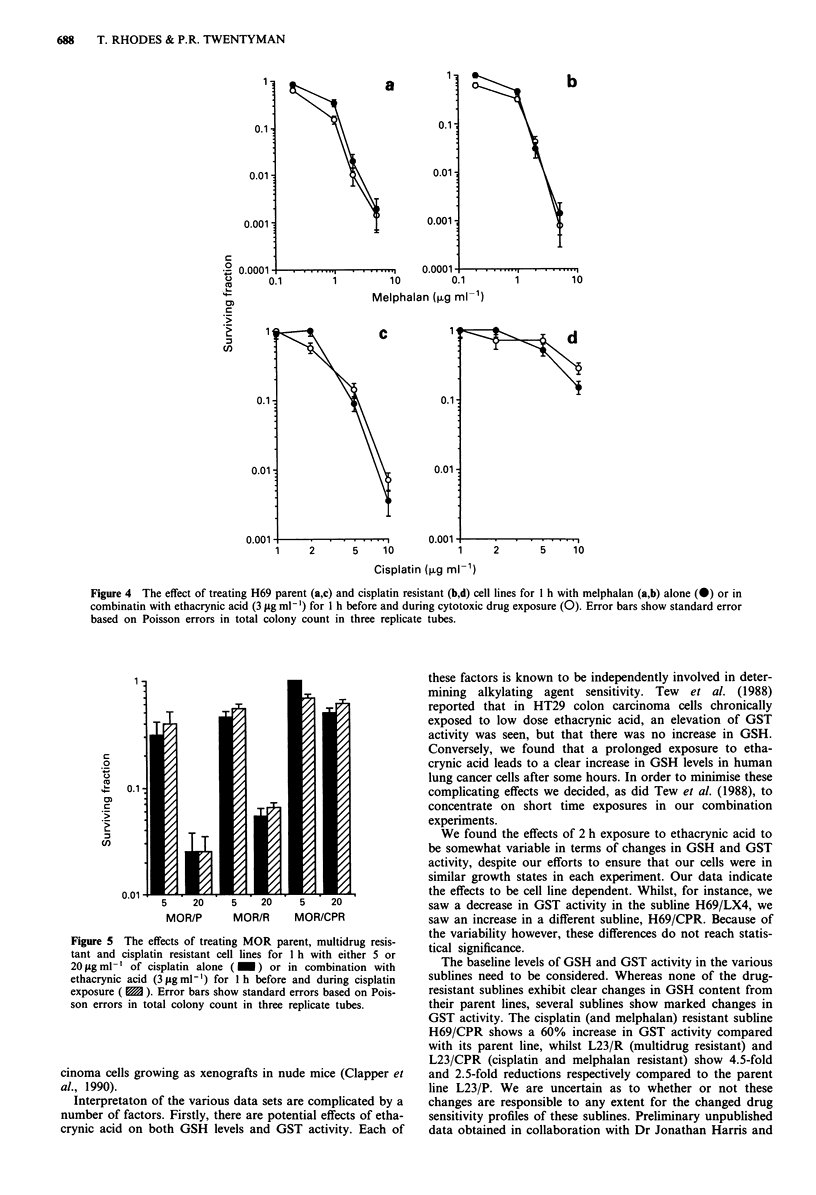
We have studied alterations in glutathione (GSH) levels and glutathione-S-transferase (GST) activity in a series of in vitro derived multidrug resistant and cisplatin resistant sublines of the human lung cancer lines NCI-H69 (small cell), COR-L23 (large cell) and MOR (adenocarcinoma). We have also investigated the effects of ethacrynic acid, a putative inhibitor of GSTs, on levels of GSH and GST activity and on cellular sensitivity to melphalan and to cisplatin. Neither GSH content nor GST activity were significantly greater in the resistant sublines compared with their respective parental lines. The only effects of treating with ethacrynic acid at doses of 1 microgram ml-1 and 3 micrograms ml-1 for 2 h were a reduction in GSH content in the cisplatin resistant subline H69/CPR at the 3 micrograms ml-1 dose, and an increase to over 140% of control at 1 microgram ml-1 and 3 micrograms ml-1 in the MOR parental line (MOR/P) and at 1 microgram ml-1 in the multidrug resistant subline MOR/R. Exposure of parental line COR-L23/P to 3 micrograms ml-1 and 6 micrograms ml-1 of ethacrynic acid for 24 h, however, increased the GSH content to over 300% and 500% of control respectively. Variable effects of ethacrynic acid on GST activity were seen in these cell lines. Doses of 1 microgram ml-1 and 3 micrograms ml-1 reduced activity to 59% and 48% of control respectively in multidrug resistant subline H69/LX4. On the other hand, activity was increased in the cisplatin resistant subline H69/CPR (to 146% and 218% of control) and in MOR/P (to 117% and 137% of control) by 1 microgram ml-1 and 3 micrograms ml-1 respectively of ethacrynic acid. Addition of ethacrynic acid (3 micrograms ml-1) to treatment of the cell lines with melphalan or with cisplatin did not alter the dose-response curves to these agents.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrick B. A., Nathan C. F. Glutathione metabolism as a determinant of therapeutic efficacy: a review. Cancer Res. 1984 Oct;44(10):4224–4232. [PubMed] [Google Scholar]
- Baillie-Johnson H., Twentyman P. R., Fox N. E., Walls G. A., Workman P., Watson J. V., Johnson N., Reeve J. G., Bleehen N. M. Establishment and characterisation of cell lines from patients with lung cancer (predominantly small cell carcinoma). Br J Cancer. 1985 Oct;52(4):495–504. doi: 10.1038/bjc.1985.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batist G., Behrens B. C., Makuch R., Hamilton T. C., Katki A. G., Louie K. G., Myers C. E., Ozols R. F. Serial determinations of glutathione levels and glutathione-related enzyme activities in human tumor cells in vitro. Biochem Pharmacol. 1986 Jul 1;35(13):2257–2259. doi: 10.1016/0006-2952(86)90601-5. [DOI] [PubMed] [Google Scholar]
- Black S. M., Beggs J. D., Hayes J. D., Bartoszek A., Muramatsu M., Sakai M., Wolf C. R. Expression of human glutathione S-transferases in Saccharomyces cerevisiae confers resistance to the anticancer drugs adriamycin and chlorambucil. Biochem J. 1990 Jun 1;268(2):309–315. doi: 10.1042/bj2680309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Connors T. A. Protection against the toxicity of alkylating agents by thiols: the mechanism of protection and its relevance to cancer chemotherapy. A review. Eur J Cancer. 1966 Dec;2(4):293–305. doi: 10.1016/0014-2964(66)90042-9. [DOI] [PubMed] [Google Scholar]
- Courtenay V. D., Mills J. An in vitro colony assay for human tumours grown in immune-suppressed mice and treated in vivo with cytotoxic agents. Br J Cancer. 1978 Feb;37(2):261–268. doi: 10.1038/bjc.1978.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedon P. C., Borch R. F. Characterization of the reactions of platinum antitumor agents with biologic and nonbiologic sulfur-containing nucleophiles. Biochem Pharmacol. 1987 Jun 15;36(12):1955–1964. doi: 10.1016/0006-2952(87)90494-1. [DOI] [PubMed] [Google Scholar]
- Dulik D. M., Fenselau C., Hilton J. Characterization of melphalan-glutathione adducts whose formation is catalyzed by glutathione transferases. Biochem Pharmacol. 1986 Oct 1;35(19):3405–3409. doi: 10.1016/0006-2952(86)90444-2. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hamilton T. C., Winker M. A., Louie K. G., Batist G., Behrens B. C., Tsuruo T., Grotzinger K. R., McKoy W. M., Young R. C., Ozols R. F. Augmentation of adriamycin, melphalan, and cisplatin cytotoxicity in drug-resistant and -sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochem Pharmacol. 1985 Jul 15;34(14):2583–2586. doi: 10.1016/0006-2952(85)90551-9. [DOI] [PubMed] [Google Scholar]
- Hansson J., Berhane K., Castro V. M., Jungnelius U., Mannervik B., Ringborg U. Sensitization of human melanoma cells to the cytotoxic effect of melphalan by the glutathione transferase inhibitor ethacrynic acid. Cancer Res. 1991 Jan 1;51(1):94–98. [PubMed] [Google Scholar]
- Leyland-Jones B. R., Townsend A. J., Tu C. P., Cowan K. H., Goldsmith M. E. Antineoplastic drug sensitivity of human MCF-7 breast cancer cells stably transfected with a human alpha class glutathione S-transferase gene. Cancer Res. 1991 Jan 15;51(2):587–594. [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- McGown A. T., Fox B. W. A proposed mechanism of resistance to cyclophosphamide and phosphoramide mustard in a Yoshida cell line in vitro. Cancer Chemother Pharmacol. 1986;17(3):223–226. doi: 10.1007/BF00256688. [DOI] [PubMed] [Google Scholar]
- Meister A., Griffith O. W. Effects of methionine sulfoximine analogs on the synthesis of glutamine and glutathione: possible chemotherapeutic implications. Cancer Treat Rep. 1979 Jun;63(6):1115–1121. [PubMed] [Google Scholar]
- Moscow J. A., Cowan K. H. Multidrug resistance. J Natl Cancer Inst. 1988 Mar 2;80(1):14–20. doi: 10.1093/jnci/80.1.14. [DOI] [PubMed] [Google Scholar]
- Moscow J. A., Townsend A. J., Cowan K. H. Elevation of pi class glutathione S-transferase activity in human breast cancer cells by transfection of the GST pi gene and its effect on sensitivity to toxins. Mol Pharmacol. 1989 Jul;36(1):22–28. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nagourney R. A., Messenger J. C., Kern D. H., Weisenthal L. M. Enhancement of anthracycline and alkylator cytotoxicity by ethacrynic acid in primary cultures of human tissues. Cancer Chemother Pharmacol. 1990;26(5):318–322. doi: 10.1007/BF02897285. [DOI] [PubMed] [Google Scholar]
- Nakagawa K., Saijo N., Tsuchida S., Sakai M., Tsunokawa Y., Yokota J., Muramatsu M., Sato K., Terada M., Tew K. D. Glutathione-S-transferase pi as a determinant of drug resistance in transfectant cell lines. J Biol Chem. 1990 Mar 15;265(8):4296–4301. [PubMed] [Google Scholar]
- Puchalski R. B., Fahl W. E. Expression of recombinant glutathione S-transferase pi, Ya, or Yb1 confers resistance to alkylating agents. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2443–2447. doi: 10.1073/pnas.87.7.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. G., Rabbitts P. H., Twentyman P. R. Non-P-glycoprotein-mediated multidrug resistance with reduced EGF receptor expression in a human large cell lung cancer cell line. Br J Cancer. 1990 Jun;61(6):851–855. doi: 10.1038/bjc.1990.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzukake K., Petro B. J., Vistica D. T. Reduction in glutathione content of L-PAM resistant L1210 Cells confers drug sensitivity. Biochem Pharmacol. 1982 Jan 1;31(1):121–124. doi: 10.1016/0006-2952(82)90249-0. [DOI] [PubMed] [Google Scholar]
- Taylor C. W., Brattain M. G., Yeoman L. C. Occurrence of cytosolic protein and phosphoprotein changes in human colon tumor cells with the development of resistance to mitomycin C. Cancer Res. 1985 Sep;45(9):4422–4427. [PubMed] [Google Scholar]
- Tew K. D., Bomber A. M., Hoffman S. J. Ethacrynic acid and piriprost as enhancers of cytotoxicity in drug resistant and sensitive cell lines. Cancer Res. 1988 Jul 1;48(13):3622–3625. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R., Fox N. E., Wright K. A., Bleehen N. M. Derivation and preliminary characterisation of adriamycin resistant lines of human lung cancer cells. Br J Cancer. 1986 Apr;53(4):529–537. doi: 10.1038/bjc.1986.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twentyman P. R., Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer. 1987 Sep;56(3):279–285. doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls G. A., Twentyman P. R. Cloning of human lung cancer cells. Br J Cancer. 1985 Oct;52(4):505–513. doi: 10.1038/bjc.1985.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. L., Tew K. D. Increased glutathione-S-transferase activity in a cell line with acquired resistance to nitrogen mustards. Cancer Treat Rep. 1985 Jun;69(6):677–682. [PubMed] [Google Scholar]
- Wang Y. Y., Teicher B. A., Shea T. C., Holden S. A., Rosbe K. W., al-Achi A., Henner W. D. Cross-resistance and glutathione-S-transferase-pi levels among four human melanoma cell lines selected for alkylating agent resistance. Cancer Res. 1989 Nov 15;49(22):6185–6192. [PubMed] [Google Scholar]
- Waxman D. J. Glutathione S-transferases: role in alkylating agent resistance and possible target for modulation chemotherapy--a review. Cancer Res. 1990 Oct 15;50(20):6449–6454. [PubMed] [Google Scholar]


