Abstract
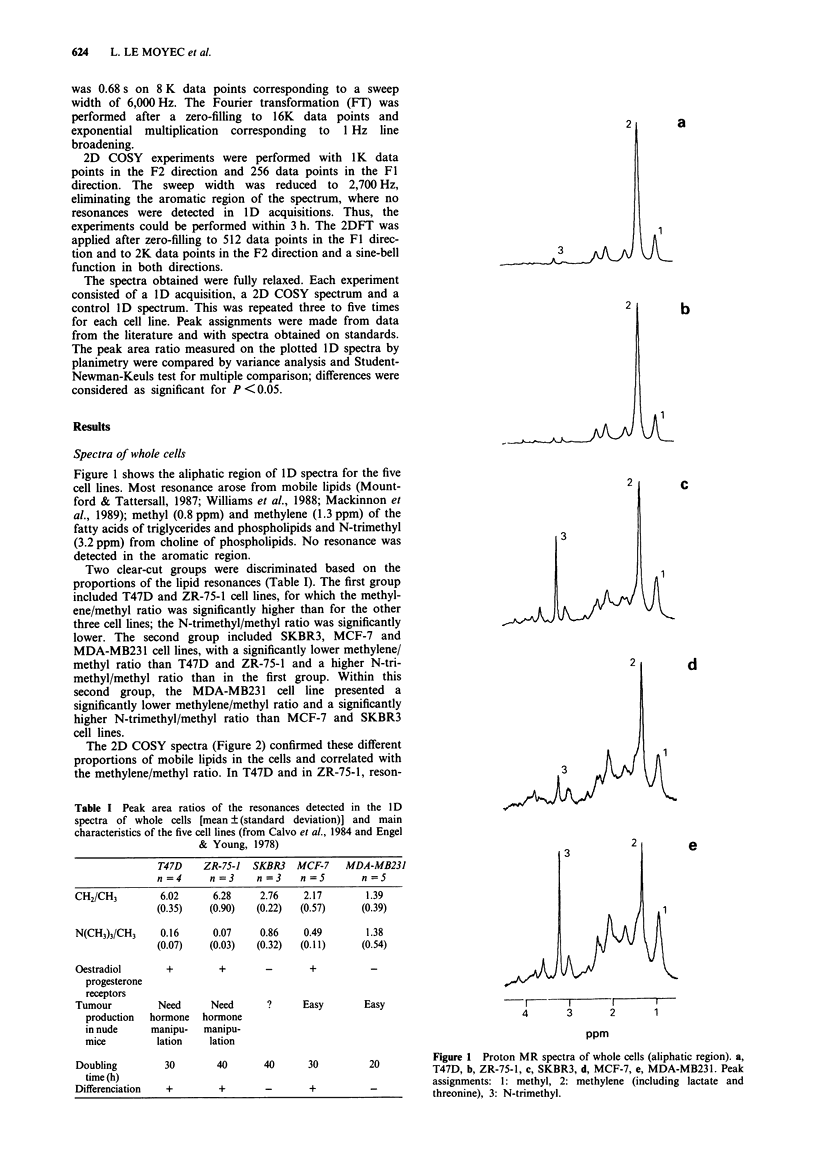
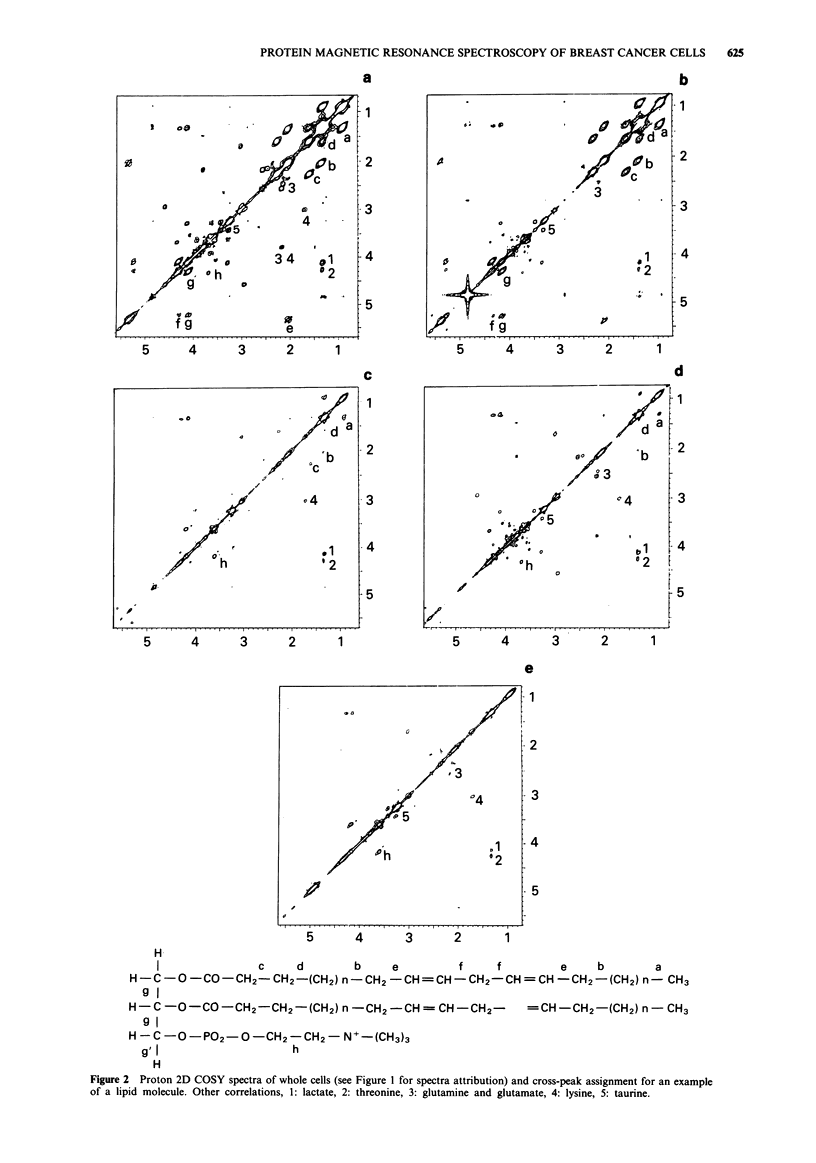
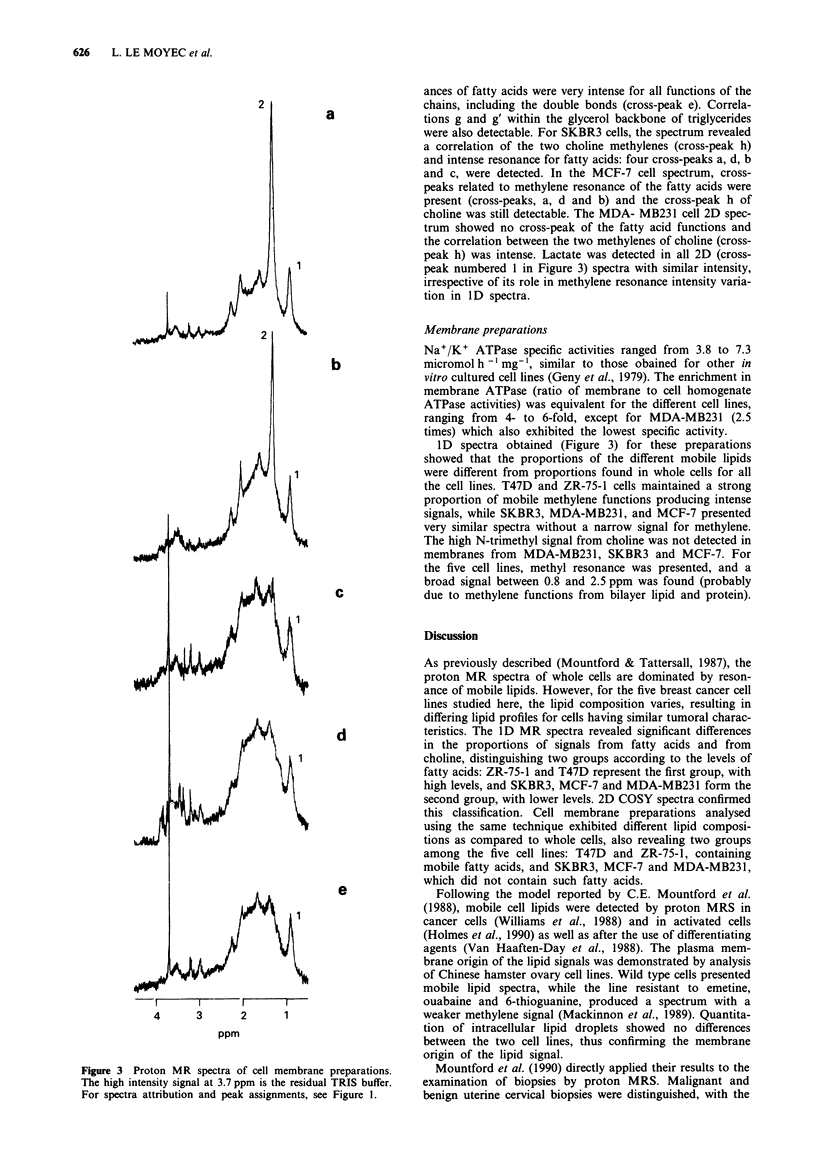
The lipid composition of five human breast cancer cell lines (MCF-7, T47D, ZR-75-1, SKBR3 and MDA-MB231) was assessed by proton magnetic resonance spectroscopy (MRS) in whole cells and membrane-enriched fractions. The proportions of the three main lipid resonances in 1D spectra were different for each cell line. These resonances included mobile methyl and methylene functions from fatty acids of triglycerides and phospholipids and N-trimethyl from choline of phospholipids. T47D and ZR-75-1 cells presented a high methylene/methyl ratio (6.02 +/- 0.35 and 6.28 +/- 0.90). This ratio was significantly lower for SKBR3, MCF-7 and MDA-MB231 cells (2.76 +/- 0.22, 2.27 +/- 0.57 and 1.39 +/- 0.39). The N-trimethyl/methyl ratio was high for MDA-MB231 and SKBR3 cells (1.38 +/- 0.54 and 0.86 +/- 0.32), but lower for MCF-7, T47D and ZR-75-1 cells (0.49 +/- 0.11, 0.16 +/- 0.07 and 0.07 +/- 0.03). 2D COSY spectra confirmed these different proportions in mobile lipids. From 1D spectra obtained on membrane preparations, T47D and ZR-75-1 were the only cell lines to retain a signal from mobile methylene functions. These differences might be related to the heterogeneity found for several parameters of these cells (tumorigenicity, growth rate, hormone receptors); an extended number of cases from fresh samples might enable clinical correlations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell J. D., Sadler P. J., Macleod A. F., Turner P. R., La Ville A. 1H NMR studies of human blood plasma. Assignment of resonances for lipoproteins. FEBS Lett. 1987 Jul 13;219(1):239–243. doi: 10.1016/0014-5793(87)81224-3. [DOI] [PubMed] [Google Scholar]
- Calvo F., Brower M., Carney D. N. Continuous culture and soft agarose cloning of multiple human breast carcinoma cell lines in serum-free medium. Cancer Res. 1984 Oct;44(10):4553–4559. [PubMed] [Google Scholar]
- Czuba M., Smith I. C. Biological and NMR markers for cancer. Pharmacol Ther. 1991;50(2):147–190. doi: 10.1016/0163-7258(91)90013-c. [DOI] [PubMed] [Google Scholar]
- Engel L. W., Young N. A. Human breast carcinoma cells in continuous culture: a review. Cancer Res. 1978 Nov;38(11 Pt 2):4327–4339. [PubMed] [Google Scholar]
- Escriba P. V., Ferrer-Montiel A. V., Ferragut J. A., Gonzalez-Ros J. M. Role of membrane lipids in the interaction of daunomycin with plasma membranes from tumor cells: implications in drug-resistance phenomena. Biochemistry. 1990 Aug 7;29(31):7275–7282. doi: 10.1021/bi00483a017. [DOI] [PubMed] [Google Scholar]
- Geny B., Lelievre L., Charlemagne D., Paraf A. Plasma membrane studies on drug sensitive and resistant cell lines. IV. Rubidium transport and ouabain binding. Exp Cell Res. 1979 May;120(2):383–393. doi: 10.1016/0014-4827(79)90398-7. [DOI] [PubMed] [Google Scholar]
- Holmes K. T., Lean C. L., Hunt N. H., King N. J. Development of the "activated" high resolution 1H MR spectrum in murine T cells and B cells occurs in G1 phase of the cell cycle. Magn Reson Med. 1990 Oct;16(1):1–8. doi: 10.1002/mrm.1910160102. [DOI] [PubMed] [Google Scholar]
- Kaplan O., Navon G., Lyon R. C., Faustino P. J., Straka E. J., Cohen J. S. Effects of 2-deoxyglucose on drug-sensitive and drug-resistant human breast cancer cells: toxicity and magnetic resonance spectroscopy studies of metabolism. Cancer Res. 1990 Feb 1;50(3):544–551. [PubMed] [Google Scholar]
- Lanson M., Bougnoux P., Besson P., Lansac J., Hubert B., Couet C., Le Floch O. N-6 polyunsaturated fatty acids in human breast carcinoma phosphatidylethanolamine and early relapse. Br J Cancer. 1990 May;61(5):776–778. doi: 10.1038/bjc.1990.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon W. B., Dyne M., Holmes K. T., Mountford C. E., Gupta R. S. Further evidence that the narrow 1H magnetic resonance signals from malignant cells do not arise from intracellular lipid droplets. NMR Biomed. 1989 Nov;2(4):161–164. doi: 10.1002/nbm.1940020405. [DOI] [PubMed] [Google Scholar]
- Mountford C. E., Delikatny E. J., Dyne M., Holmes K. T., Mackinnon W. B., Ford R., Hunter J. C., Truskett I. D., Russell P. Uterine cervical punch biopsy specimens can be analyzed by 1H MRS. Magn Reson Med. 1990 Feb;13(2):324–331. doi: 10.1002/mrm.1910130216. [DOI] [PubMed] [Google Scholar]
- Mountford C. E., Tattersall M. H. Proton magnetic resonance spectroscopy and tumour detection. Cancer Surv. 1987;6(2):285–314. [PubMed] [Google Scholar]
- Mountford C. E., Wright L. C. Organization of lipids in the plasma membranes of malignant and stimulated cells: a new model. Trends Biochem Sci. 1988 May;13(5):172–177. doi: 10.1016/0968-0004(88)90145-4. [DOI] [PubMed] [Google Scholar]
- Noel F., Godfraind T. Heterogeneity of ouabain specific binding sites and (Na+ + K+)-ATPase inhibition in microsomes from rat heart. Biochem Pharmacol. 1984 Jan 1;33(1):47–53. doi: 10.1016/0006-2952(84)90369-1. [DOI] [PubMed] [Google Scholar]
- Reibnegger G., Weiss G., Werner-Felmayer G., Judmaier G., Wachter H. Neural networks as a tool for utilizing laboratory information: comparison with linear discriminant analysis and with classification and regression trees. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11426–11430. doi: 10.1073/pnas.88.24.11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynier M., Sari H., d'Anglebermes M., Kye E. A., Pasero L. Differences in lipid characteristics of undifferentiated and enterocytic-differentiated HT29 human colonic cells. Cancer Res. 1991 Feb 15;51(4):1270–1277. [PubMed] [Google Scholar]
- Van Zijl P. C., Moonen C. T., Faustino P., Pekar J., Kaplan O., Cohen J. S. Complete separation of intracellular and extracellular information in NMR spectra of perfused cells by diffusion-weighted spectroscopy. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3228–3232. doi: 10.1073/pnas.88.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. G., Saunders J. K., Dyne M., Mountford C. E., Holmes K. T. Application of a T2-filtered COSY experiment to identify the origin of slowly relaxing species in normal and malignant tissue. Magn Reson Med. 1988 Aug;7(4):463–471. doi: 10.1002/mrm.1910070409. [DOI] [PubMed] [Google Scholar]
- Zajchowski D., Band V., Pauzie N., Tager A., Stampfer M., Sager R. Expression of growth factors and oncogenes in normal and tumor-derived human mammary epithelial cells. Cancer Res. 1988 Dec 15;48(24 Pt 1):7041–7047. [PubMed] [Google Scholar]


