Abstract
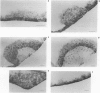
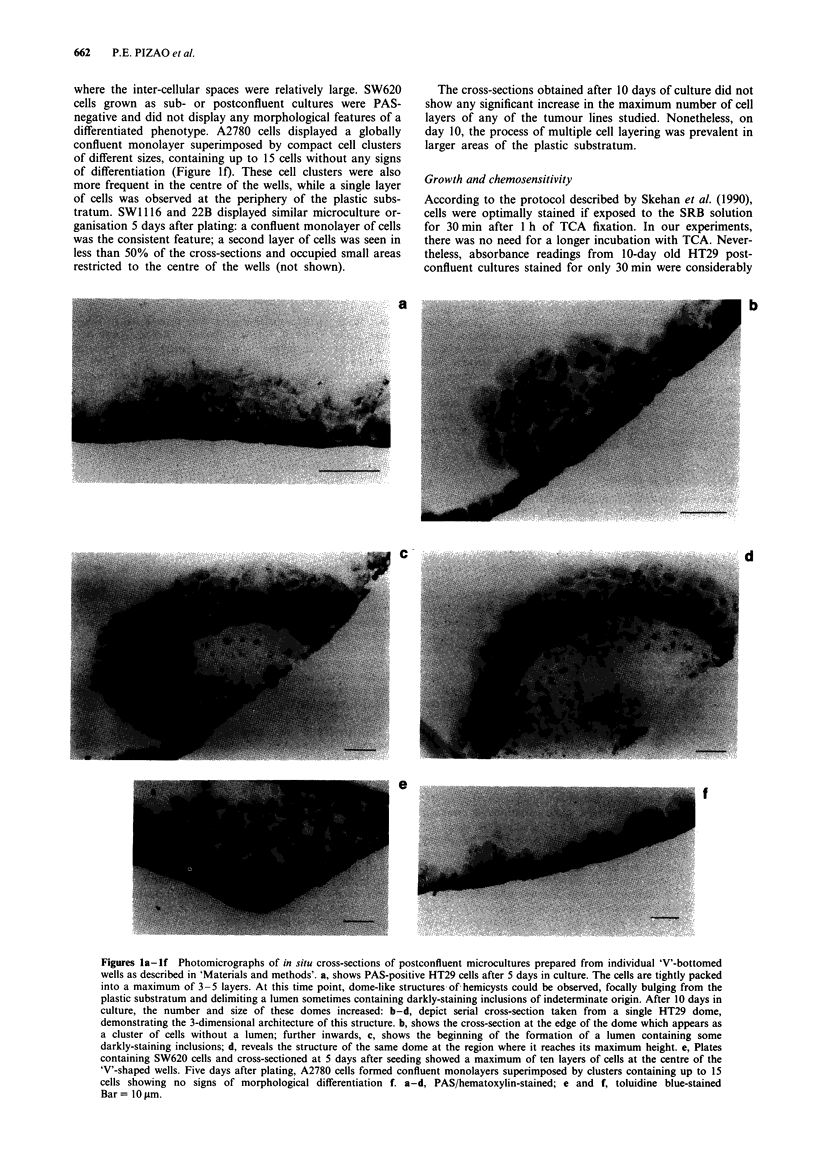
This study assessed the growth pattern, cellular organisation and chemosensitivity of established human tumour cell lines growing as postconfluent cultures in 'V'-bottomed, 96-well microtiter plates. Cross-sections of the colon (HT29, SW620, SW1116), ovarian (A2780) and head and neck (UM-SCC-22B) carcinoma microcultures allowed in situ evaluation of the cellular organisation in the wells. After 5 days of growth, every cell line had reached confluence, but each of them displayed a specific pattern of cell stacking which ranged from two to ten layers. Postconfluent HT29 cells displayed morphologic features suggestive of some degree of enterocytic differentiation. Growth and cytotoxicity could be studied reliably and reproducibly in this system with the sulforhodamine B protein assay. Against HT29 postconfluent cultures, the EC50's (drug concentrations producing absorbance readings 50% lower than those of non-treated wells) of 5-fluorouracil and of the ether lipid, hexadecylphosphocholine, were 1 mM and 50 microM respectively. The possibility to perform chemosensitivity tests using semiautomated microtiter plate technology supports further evaluation of this system as an alternative antitumour drug testing model.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alley M. C., Scudiero D. A., Monks A., Hursey M. L., Czerwinski M. J., Fine D. L., Abbott B. J., Mayo J. G., Shoemaker R. H., Boyd M. R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988 Feb 1;48(3):589–601. [PubMed] [Google Scholar]
- Anai H., Maehara Y., Kusumoto H., Sugimachi K. Comparison between succinate dehydrogenase inhibition test and subrenal capsule assay for chemosensitivity testing. Oncology. 1987;44(2):115–117. doi: 10.1159/000226457. [DOI] [PubMed] [Google Scholar]
- Arnould R., Dubois J., Abikhalil F., Libert A., Ghanem G., Atassi G., Hanocq M., Lejeune F. J. Comparison of two cytotoxicity assays--tetrazolium derivative reduction (MTT) and tritiated thymidine uptake--on three malignant mouse cell lines using chemotherapeutic agents and investigational drugs. Anticancer Res. 1990 Jan-Feb;10(1):145–154. [PubMed] [Google Scholar]
- Chantret I., Barbat A., Dussaulx E., Brattain M. G., Zweibaum A. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res. 1988 Apr 1;48(7):1936–1942. [PubMed] [Google Scholar]
- Daneker G. W., Jr, Piazza A. J., Steele G. D., Jr, Mercurio A. M. Relationship between extracellular matrix interactions and degree of differentiation in human colon carcinoma cell lines. Cancer Res. 1989 Feb 1;49(3):681–686. [PubMed] [Google Scholar]
- Dertinger H., Guichard M., Malaise E. P. Relationship between intercellular communication and radiosensitivity of human tumor xenografts. Eur J Cancer Clin Oncol. 1984 Apr;20(4):561–566. doi: 10.1016/0277-5379(84)90243-8. [DOI] [PubMed] [Google Scholar]
- Drewinko B., Patchen M., Yang L. Y., Barlogie B. Differential killing efficacy of twenty antitumor drugs on proliferating and nonproliferating human tumor cells. Cancer Res. 1981 Jun;41(6):2328–2333. [PubMed] [Google Scholar]
- Fantini J., Abadie B., Tirard A., Remy L., Ripert J. P., el Battari A., Marvaldi J. Spontaneous and induced dome formation by two clonal cell populations derived from a human adenocarcinoma cell line, HT29. J Cell Sci. 1986 Jul;83:235–249. doi: 10.1242/jcs.83.1.235. [DOI] [PubMed] [Google Scholar]
- Garrouste F., Remacle-Bonnet M., Culouscou J. M., Marvaldi J., Pommier G. Type-II insulin-like growth-factor receptor in conditioned medium from HT-29 human colon carcinoma cell line. Int J Cancer. 1991 Mar 12;47(5):760–764. doi: 10.1002/ijc.2910470523. [DOI] [PubMed] [Google Scholar]
- Hafez M. M., Infante D., Winawer S., Friedman E. Transforming growth factor beta 1 acts as an autocrine-negative growth regulator in colon enterocytic differentiation but not in goblet cell maturation. Cell Growth Differ. 1990 Dec;1(12):617–626. [PubMed] [Google Scholar]
- Heo D. S., Park J. G., Hata K., Day R., Herberman R. B., Whiteside T. L. Evaluation of tetrazolium-based semiautomatic colorimetric assay for measurement of human antitumor cytotoxicity. Cancer Res. 1990 Jun 15;50(12):3681–3690. [PubMed] [Google Scholar]
- Hilgard P., Stekar J., Voegeli R., Engel J., Schumacher W., Eibl H., Unger C., Berger M. R. Characterization of the antitumor activity of hexadecylphosphocholine (D 18506). Eur J Cancer Clin Oncol. 1988 Sep;24(9):1457–1461. doi: 10.1016/0277-5379(88)90336-7. [DOI] [PubMed] [Google Scholar]
- Hoffman R. M. In vitro sensitivity assays in cancer: a review, analysis, and prognosis. J Clin Lab Anal. 1991;5(2):133–143. doi: 10.1002/jcla.1860050211. [DOI] [PubMed] [Google Scholar]
- Keepers Y. P., Pizao P. E., Peters G. J., van Ark-Otte J., Winograd B., Pinedo H. M. Comparison of the sulforhodamine B protein and tetrazolium (MTT) assays for in vitro chemosensitivity testing. Eur J Cancer. 1991;27(7):897–900. doi: 10.1016/0277-5379(91)90142-z. [DOI] [PubMed] [Google Scholar]
- Kondo T., Imamura T., Ichihashi H. In vitro test for sensitivity of tumor to carcinostatic agents. Gan. 1966 Apr;57(2):113–121. [PubMed] [Google Scholar]
- Kruse P. F., Jr, Miedema E. Production and characterization of multiple-layered populations of animal cells. J Cell Biol. 1965 Nov;27(2):273–279. doi: 10.1083/jcb.27.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik H. Z., Collins E. A., O'Brien M. J., McCaffrey R. P. Chemotherapy screening assay using 3-dimensional cell culture. Cancer Lett. 1990 May 15;51(1):11–16. doi: 10.1016/0304-3835(90)90224-l. [DOI] [PubMed] [Google Scholar]
- Lesuffleur T., Barbat A., Dussaulx E., Zweibaum A. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 1990 Oct 1;50(19):6334–6343. [PubMed] [Google Scholar]
- Lesuffleur T., Kornowski A., Augeron C., Dussaulx E., Barbat A., Laboisse C., Zweibaum A. Increased growth adaptability to 5-fluorouracil and methotrexate of HT-29 sub-populations selected for their commitment to differentiation. Int J Cancer. 1991 Nov 11;49(5):731–737. doi: 10.1002/ijc.2910490517. [DOI] [PubMed] [Google Scholar]
- Maehara Y., Anai H., Kusumoto H., Sugimachi K. Poorly differentiated human gastric carcinoma is more sensitive to antitumor drugs than is well differentiated carcinoma. Eur J Surg Oncol. 1987 Jun;13(3):203–206. [PubMed] [Google Scholar]
- Maehara Y., Anai H., Tamada R., Sugimachi K. The ATP assay is more sensitive than the succinate dehydrogenase inhibition test for predicting cell viability. Eur J Cancer Clin Oncol. 1987 Mar;23(3):273–276. doi: 10.1016/0277-5379(87)90070-8. [DOI] [PubMed] [Google Scholar]
- Mitsudomi T., Kaneko S., Tateishi M., Yano T., Ishida T., Kohnoe S., Maehara Y., Sugimachi K. Chemosensitivity testing of human lung cancer tissues using the succinate dehydrogenase inhibition test. Anticancer Res. 1990 Jul-Aug;10(4):987–990. [PubMed] [Google Scholar]
- Mueller-Klieser W. Multicellular spheroids. A review on cellular aggregates in cancer research. J Cancer Res Clin Oncol. 1987;113(2):101–122. doi: 10.1007/BF00391431. [DOI] [PubMed] [Google Scholar]
- Osieka R., Houchens D. P., Goldin A., Johnson R. K. Chemotherapy of human colon cancer xenografts in athymic nude mice. Cancer. 1977 Nov;40(5 Suppl):2640–2650. doi: 10.1002/1097-0142(197711)40:5+<2640::aid-cncr2820400938>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pelletier H., Millot J. M., Chauffert B., Manfait M., Genne P., Martin F. Mechanisms of resistance of confluent human and rat colon cancer cells to anthracyclines: alteration of drug passive diffusion. Cancer Res. 1990 Oct 15;50(20):6626–6631. [PubMed] [Google Scholar]
- Phillips R. M., Bibby M. C., Double J. A. A critical appraisal of the predictive value of in vitro chemosensitivity assays. J Natl Cancer Inst. 1990 Sep 19;82(18):1457–1468. doi: 10.1093/jnci/82.18.1457. [DOI] [PubMed] [Google Scholar]
- Richman P. I., Bodmer W. F. Monoclonal antibodies to human colorectal epithelium: markers for differentiation and tumour characterization. Int J Cancer. 1987 Mar 15;39(3):317–328. doi: 10.1002/ijc.2910390309. [DOI] [PubMed] [Google Scholar]
- Ruben R. L., Neubauer R. H. Semiautomated colorimetric assay for in vitro screening of anticancer compounds. Cancer Treat Rep. 1987 Dec;71(12):1141–1149. [PubMed] [Google Scholar]
- Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J. T., Bokesch H., Kenney S., Boyd M. R. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990 Jul 4;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Skehan P., Thomas J., Friedman S. J. Postconfluency MDCK monolayers as an in vitro model of solid tumor chemosensitivity. Cell Biol Toxicol. 1986 Sep;2(3):357–368. doi: 10.1007/BF00121851. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988 Apr 8;240(4849):177–184. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R. Comparative chemosensitivity of exponential- versus plateau-phase cells in both in vitro model systems. Cancer Treat Rep. 1976 Dec;60(12):1719–1722. [PubMed] [Google Scholar]
- Twentyman P. R., Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer. 1987 Sep;56(3):279–285. doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viallard V., Denis C., Trocheris V., Murat J. C. Effect of glutamine deprivation and glutamate or ammonium chloride addition on growth rate, metabolism and differentiation of human colon cancer cell-line HT29. Int J Biochem. 1986;18(3):263–269. doi: 10.1016/0020-711x(86)90116-3. [DOI] [PubMed] [Google Scholar]
- Yuhas J. M., Tarleton A. E., Molzen K. B. Multicellular tumor spheroid formation by breast cancer cells isolated from different sites. Cancer Res. 1978 Aug;38(8):2486–2491. [PubMed] [Google Scholar]