Abstract
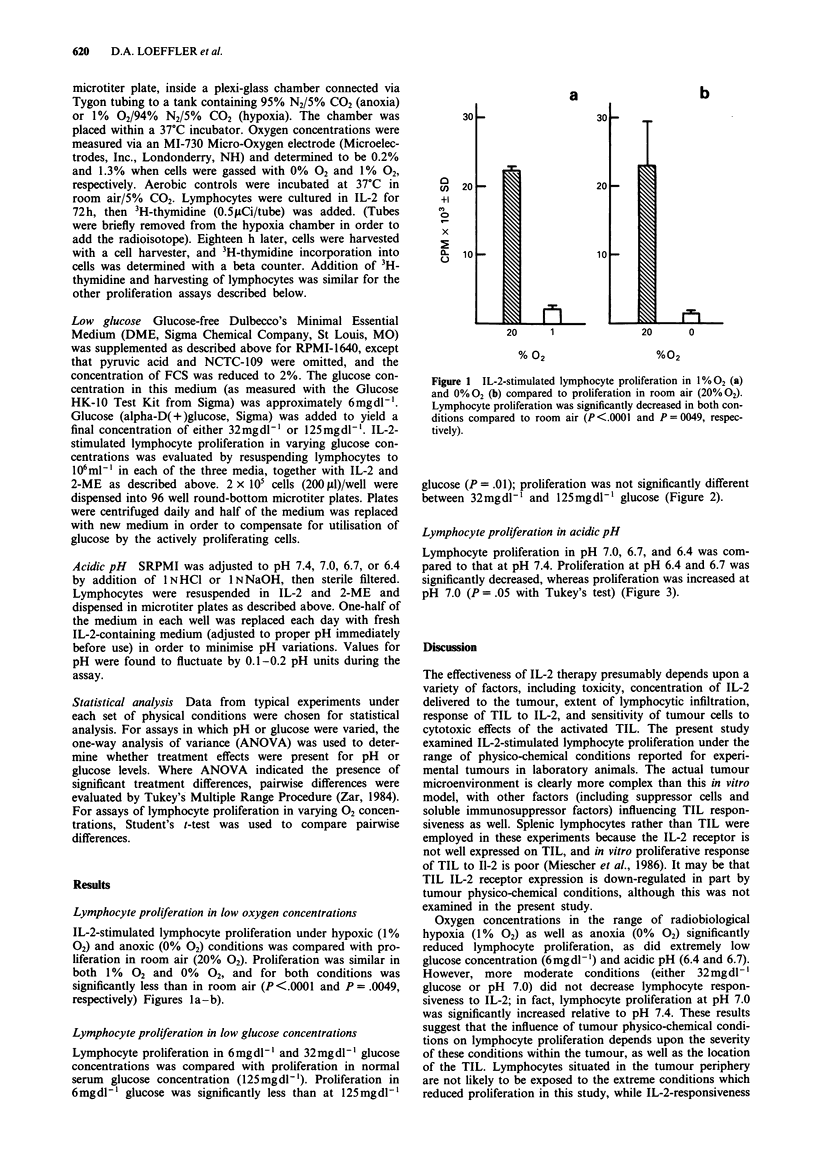
The proliferative response of murine lymphocytes to interleukin-2 (IL-2) was examined under physico-chemical conditions present in solid tumours, namely low oxygen and glucose concentrations and acidic pH. Lymphocytes were cultured for four days in 30 U ml-1 IL-2 to simulate serum IL-2 concentrations attainable with high-dose systemic IL-2 therapy. Lymphocyte proliferation was significantly (P < 0.05) reduced by low oxygen concentrations (both anoxia [0% O2] and hypoxia [10%, low glucose (6 mg dl-1), or acidic pH (6.7 or 6.4). Moderate glucose concentration (32 mg dl-1), or neutral pH (7.0) did not impair proliferation. This study indicates that impairment of lymphocyte proliferation by tumour physico-chemical conditions may be a factor in the relatively poor success rate of IL-2/LAK cell immunotherapy.
Full text
PDF
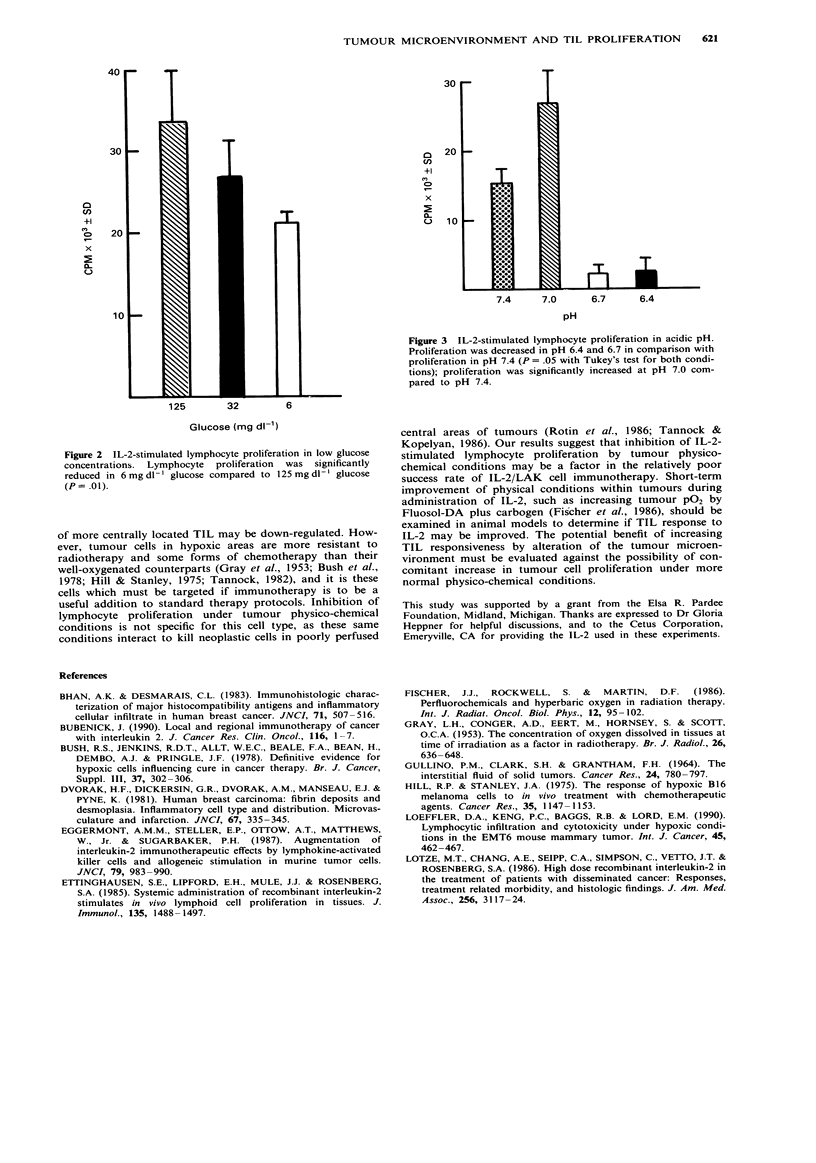

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhan A. K., DesMarais C. L. Immunohistologic characterization of major histocompatibility antigens and inflammatory cellular infiltrate in human breast cancer. J Natl Cancer Inst. 1983 Sep;71(3):507–516. [PubMed] [Google Scholar]
- Bush R. S., Jenkin R. D., Allt W. E., Beale F. A., Bean H., Dembo A. J., Pringle J. F. Definitive evidence for hypoxic cells influencing cure in cancer therapy. Br J Cancer Suppl. 1978 Jun;3:302–306. [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Dickersin G. R., Dvorak A. M., Manseau E. J., Pyne K. Human breast carcinoma: fibrin deposits and desmoplasia. Inflammatory cell type and distribution. Microvasculature and infarction. J Natl Cancer Inst. 1981 Aug;67(2):335–345. [PubMed] [Google Scholar]
- Eggermont A. M., Steller E. P., Ottow R. T., Matthews W., Jr, Sugarbaker P. H. Augmentation of interleukin-2 immunotherapeutic effects by lymphokine-activated killer cells and allogeneic stimulation in murine tumor cells. J Natl Cancer Inst. 1987 Nov;79(5):983–990. [PubMed] [Google Scholar]
- Ettinghausen S. E., Lipford E. H., 3rd, Mulé J. J., Rosenberg S. A. Systemic administration of recombinant interleukin 2 stimulates in vivo lymphoid cell proliferation in tissues. J Immunol. 1985 Aug;135(2):1488–1497. [PubMed] [Google Scholar]
- Fischer J. J., Rockwell S., Martin D. F. Perfluorochemicals and hyperbaric oxygen in radiation therapy. Int J Radiat Oncol Biol Phys. 1986 Jan;12(1):95–102. doi: 10.1016/0360-3016(86)90421-9. [DOI] [PubMed] [Google Scholar]
- GRAY L. H., CONGER A. D., EBERT M., HORNSEY S., SCOTT O. C. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953 Dec;26(312):638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- GULLINO P. M., CLARK S. H., GRANTHAM F. H. THE INTERSTITIAL FLUID OF SOLID TUMORS. Cancer Res. 1964 Jun;24:780–794. [PubMed] [Google Scholar]
- Hill R. P., Stanley J. A. The response of hypoxic B16 melanoma cells to in vivo treatment with chemotherapeutic agents. Cancer Res. 1975 May;35(5):1147–1153. [PubMed] [Google Scholar]
- Loeffler D. A., Keng P. C., Baggs R. B., Lord E. M. Lymphocytic infiltration and cytotoxicity under hypoxic conditions in the EMT6 mouse mammary tumor. Int J Cancer. 1990 Mar 15;45(3):462–467. doi: 10.1002/ijc.2910450315. [DOI] [PubMed] [Google Scholar]
- Lotze M. T., Chang A. E., Seipp C. A., Simpson C., Vetto J. T., Rosenberg S. A. High-dose recombinant interleukin 2 in the treatment of patients with disseminated cancer. Responses, treatment-related morbidity, and histologic findings. JAMA. 1986 Dec 12;256(22):3117–3124. [PubMed] [Google Scholar]
- Lotze M. T., Line B. R., Mathisen D. J., Rosenberg S. A. The in vivo distribution of autologous human and murine lymphoid cells grown in T cell growth factor (TCGF): implications for the adoptive immunotherapy of tumors. J Immunol. 1980 Oct;125(4):1487–1493. [PubMed] [Google Scholar]
- Mertelsmann R., Welte K. Human interleukin 2: molecular biology, physiology and clinical possibilities. Immunobiology. 1986 Sep;172(3-5):400–419. doi: 10.1016/S0171-2985(86)80121-8. [DOI] [PubMed] [Google Scholar]
- Miescher S., Whiteside T. L., Carrel S., von Fliedner V. Functional properties of tumor-infiltrating and blood lymphocytes in patients with solid tumors: effects of tumor cells and their supernatants on proliferative responses of lymphocytes. J Immunol. 1986 Mar 1;136(5):1899–1907. [PubMed] [Google Scholar]
- Oliver R. T., Crosby D., Nouri A., Scott E., Galazka A. Evaluation of the effect of continuous infusion recombinant interleukin-2 (bioleukin) on peripheral blood leucocytes of patients with terminal malignancy. Br J Cancer. 1989 Dec;60(6):934–937. doi: 10.1038/bjc.1989.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Mulé J. J., Spiess P. J., Reichert C. M., Schwarz S. L. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med. 1985 May 1;161(5):1169–1188. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin D., Robinson B., Tannock I. F. Influence of hypoxia and an acidic environment on the metabolism and viability of cultured cells: potential implications for cell death in tumors. Cancer Res. 1986 Jun;46(6):2821–2826. [PubMed] [Google Scholar]
- Ruscetti F. W., Gallo R. C. Human T-lymphocyte growth factor: regulation of growth and function of T lymphocytes. Blood. 1981 Mar;57(3):379–394. [PubMed] [Google Scholar]
- Sosman J. A., Hank J. A., Sondel P. M. In vivo activation of lymphokine-activated killer activity with interleukin-2: prospects for combination therapies. Semin Oncol. 1990 Feb;17(1 Suppl 1):22–41. [PubMed] [Google Scholar]
- Tannock I. F., Kopelyan I. Variation of pO2 in the growth medium of spheroids: interaction with glucose to influence spheroid growth and necrosis. Br J Cancer. 1986 Jun;53(6):823–827. doi: 10.1038/bjc.1986.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I. Response of aerobic and hypoxic cells in a solid tumor to adriamycin and cyclophosphamide and interaction of the drugs with radiation. Cancer Res. 1982 Dec;42(12):4921–4926. [PubMed] [Google Scholar]
- Vaage J., Pepin K. G. Morphological observations during developing concomitant immunity against a C3H/He mammary tumor. Cancer Res. 1985 Feb;45(2):659–666. [PubMed] [Google Scholar]
- Vaupel P. W., Frinak S., Bicher H. I. Heterogeneous oxygen partial pressure and pH distribution in C3H mouse mammary adenocarcinoma. Cancer Res. 1981 May;41(5):2008–2013. [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]


