Abstract
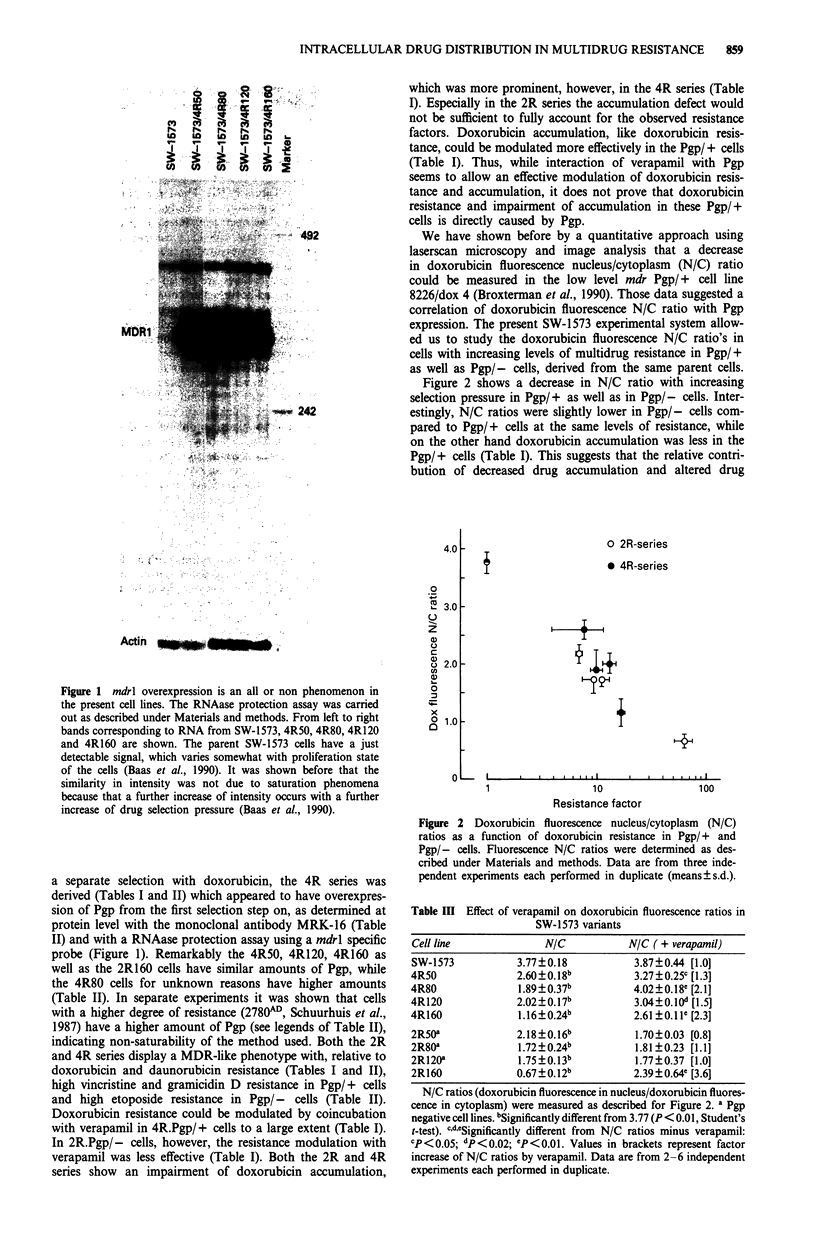
Resistance to multiple antitumour drugs, mostly antibiotics or alkaloids, has been associated with a cellular plasma membrane P-glycoprotein (Pgp), causing energy-dependent transport of drugs out of cells. However, in many common chemotherapy resistant human cancers there is no overexpression of Pgp, which could explain drug resistance. In order to characterise early steps in multidrug resistance we have derived a series of P-glycoprotein-positive (Pgp/+) and P-glycoprotein-negative (Pgp/-) multidrug resistant cell lines, from a human non-small cell lung cancer cell line, SW-1573, by stepwise selection with increasing concentrations of doxorubicin. These cells were exposed to doxorubicin and its fluorescence in nucleus (N) and cytoplasm (C) was quantified with laserscan microscopy and image analysis. The fluorescence N/C ratio in parent cells was 3.8 and decreased both in Pgp/+ and Pgp/- cells with increasing selection pressure to 1.2-2.6 for cells with a resistance factor of 7-17. N/C ratios could be restored partly with verapamil only in Pgp/+ cells. N/C ratio measurements may define a general Pgp-independent type of defense of mammalian cells against certain anticancer agents which may precede Pgp expression in early doxorubicin resistance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
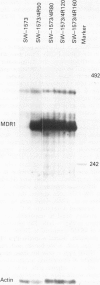
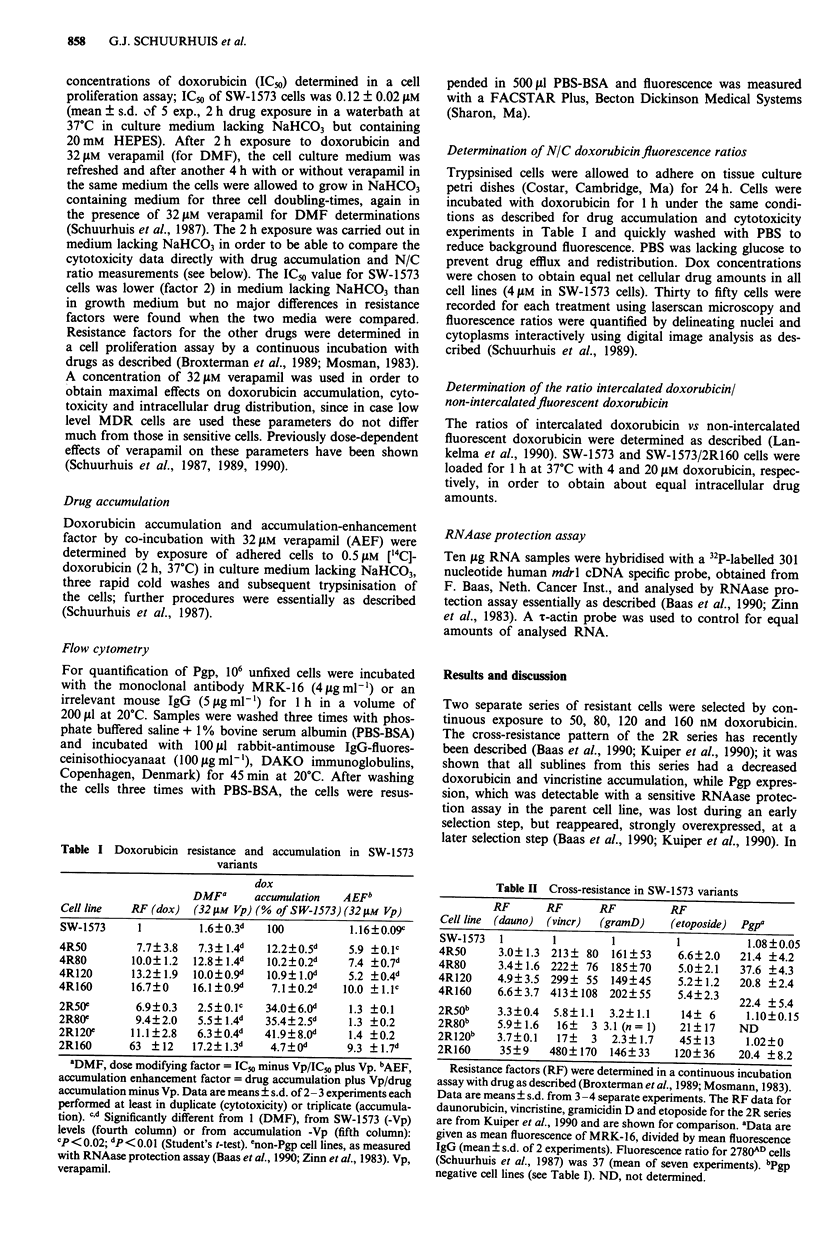
- Baas F., Jongsma A. P., Broxterman H. J., Arceci R. J., Housman D., Scheffer G. L., Riethorst A., van Groenigen M., Nieuwint A. W., Joenje H. Non-P-glycoprotein mediated mechanism for multidrug resistance precedes P-glycoprotein expression during in vitro selection for doxorubicin resistance in a human lung cancer cell line. Cancer Res. 1990 Sep 1;50(17):5392–5398. [PubMed] [Google Scholar]
- Beck W. T. The cell biology of multiple drug resistance. Biochem Pharmacol. 1987 Sep 15;36(18):2879–2887. doi: 10.1016/0006-2952(87)90198-5. [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Chang T. D., Scotto K. W., Melera P. W., Spengler B. A. Chromosomal organization of amplified genes in multidrug-resistant Chinese hamster cells. Cancer Res. 1988 Jun 1;48(11):3179–3187. [PubMed] [Google Scholar]
- Bradley G., Juranka P. F., Ling V. Mechanism of multidrug resistance. Biochim Biophys Acta. 1988 Aug 3;948(1):87–128. doi: 10.1016/0304-419x(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Broxterman H. J., Pinedo H. M., Kuiper C. M., Kaptein L. C., Schuurhuis G. J., Lankelma J. Induction by verapamil of a rapid increase in ATP consumption in multidrug-resistant tumor cells. FASEB J. 1988 Apr;2(7):2278–2282. doi: 10.1096/fasebj.2.7.3350243. [DOI] [PubMed] [Google Scholar]
- Broxterman H. J., Pinedo H. M., Kuiper C. M., van der Hoeven J. J., de Lange P., Quak J. J., Scheper R. J., Keizer H. G., Schuurhuis G. J., Lankelma J. Immunohistochemical detection of P-glycoprotein in human tumor cells with a low degree of drug resistance. Int J Cancer. 1989 Feb 15;43(2):340–343. doi: 10.1002/ijc.2910430229. [DOI] [PubMed] [Google Scholar]
- Burke T. G., Tritton T. R. Location and dynamics of anthracyclines bound to unilamellar phosphatidylcholine vesicles. Biochemistry. 1985 Oct 8;24(21):5972–5980. doi: 10.1021/bi00342a043. [DOI] [PubMed] [Google Scholar]
- Carl J. Drug-resistance patterns assessed from tumor marker analysis. J Natl Cancer Inst. 1989 Nov 1;81(21):1631–1639. doi: 10.1093/jnci/81.21.1631. [DOI] [PubMed] [Google Scholar]
- Chaires J. B., Dattagupta N., Crothers D. M. Studies on interaction of anthracycline antibiotics and deoxyribonucleic acid: equilibrium binding studies on interaction of daunomycin with deoxyribonucleic acid. Biochemistry. 1982 Aug 17;21(17):3933–3940. doi: 10.1021/bi00260a005. [DOI] [PubMed] [Google Scholar]
- Choi K. H., Chen C. J., Kriegler M., Roninson I. B. An altered pattern of cross-resistance in multidrug-resistant human cells results from spontaneous mutations in the mdr1 (P-glycoprotein) gene. Cell. 1988 May 20;53(4):519–529. doi: 10.1016/0092-8674(88)90568-5. [DOI] [PubMed] [Google Scholar]
- Coldman A. J., Goldie J. H. Role of mathematical modeling in protocol formulation in cancer chemotherapy. Cancer Treat Rep. 1985 Oct;69(10):1041–1048. [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Resistance to multiple chemotherapeutic agents in human cancer cells. Trends Pharmacol Sci. 1988 Feb;9(2):54–58. doi: 10.1016/0165-6147(88)90117-4. [DOI] [PubMed] [Google Scholar]
- Gros P., Croop J., Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986 Nov 7;47(3):371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- Hammond J. R., Johnstone R. M., Gros P. Enhanced efflux of [3H]vinblastine from Chinese hamster ovary cells transfected with a full-length complementary DNA clone for the mdr1 gene. Cancer Res. 1989 Jul 15;49(14):3867–3871. [PubMed] [Google Scholar]
- Henderson G. B., Tsuji J. M. Identification of cholate as a shared substrate for the unidirectional efflux systems for methotrexate in L1210 mouse cells. Biochim Biophys Acta. 1990 Jan 23;1051(1):60–70. doi: 10.1016/0167-4889(90)90174-c. [DOI] [PubMed] [Google Scholar]
- Hindenburg A. A., Gervasoni J. E., Jr, Krishna S., Stewart V. J., Rosado M., Lutzky J., Bhalla K., Baker M. A., Taub R. N. Intracellular distribution and pharmacokinetics of daunorubicin in anthracycline-sensitive and -resistant HL-60 cells. Cancer Res. 1989 Aug 15;49(16):4607–4614. [PubMed] [Google Scholar]
- Juranka P. F., Zastawny R. L., Ling V. P-glycoprotein: multidrug-resistance and a superfamily of membrane-associated transport proteins. FASEB J. 1989 Dec;3(14):2583–2592. doi: 10.1096/fasebj.3.14.2574119. [DOI] [PubMed] [Google Scholar]
- Keizer H. G., Joenje H. Increased cytosolic pH in multidrug-resistant human lung tumor cells: effect of verapamil. J Natl Cancer Inst. 1989 May 3;81(9):706–709. doi: 10.1093/jnci/81.9.706. [DOI] [PubMed] [Google Scholar]
- Keizer H. G., Schuurhuis G. J., Broxterman H. J., Lankelma J., Schoonen W. G., van Rijn J., Pinedo H. M., Joenje H. Correlation of multidrug resistance with decreased drug accumulation, altered subcellular drug distribution, and increased P-glycoprotein expression in cultured SW-1573 human lung tumor cells. Cancer Res. 1989 Jun 1;49(11):2988–2993. [PubMed] [Google Scholar]
- Lankelma J., Mülder H. S., van Mourik F., Wong Fong Sang H. W., Kraayenhof R., van Grondelle R. Cellular daunomycin fluorescence in multidrug resistant 2780AD cells and its relation to cellular drug localisation. Biochim Biophys Acta. 1991 Jul 10;1093(2-3):147–152. doi: 10.1016/0167-4889(91)90116-f. [DOI] [PubMed] [Google Scholar]
- Lincke C. R., van der Bliek A. M., Schuurhuis G. J., van der Velde-Koerts T., Smit J. J., Borst P. Multidrug resistance phenotype of human BRO melanoma cells transfected with a wild-type human mdr1 complementary DNA. Cancer Res. 1990 Mar 15;50(6):1779–1785. [PubMed] [Google Scholar]
- McGrath T., Latoud C., Arnold S. T., Safa A. R., Felsted R. L., Center M. S. Mechanisms of multidrug resistance in HL60 cells. Analysis of resistance associated membrane proteins and levels of mdr gene expression. Biochem Pharmacol. 1989 Oct 15;38(20):3611–3619. doi: 10.1016/0006-2952(89)90134-2. [DOI] [PubMed] [Google Scholar]
- McGrath T., Marquardt D., Center M. S. Multiple mechanisms of adriamycin resistance in the human leukemia cell line CCRF-CEM. Biochem Pharmacol. 1989 Feb 1;38(3):497–501. doi: 10.1016/0006-2952(89)90390-0. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Schuurhuis G. J., Broxterman H. J., Cervantes A., van Heijningen T. H., de Lange J. H., Baak J. P., Pinedo H. M., Lankelma J. Quantitative determination of factors contributing to doxorubicin resistance in multidrug-resistant cells. J Natl Cancer Inst. 1989 Dec 20;81(24):1887–1892. doi: 10.1093/jnci/81.24.1887. [DOI] [PubMed] [Google Scholar]
- Schuurhuis G. J., Broxterman H. J., Pinedo H. M., van Heijningen T. H., van Kalken C. K., Vermorken J. B., Spoelstra E. C., Lankelma J. The polyoxyethylene castor oil Cremophor EL modifies multidrug resistance. Br J Cancer. 1990 Oct;62(4):591–594. doi: 10.1038/bjc.1990.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurhuis G. J., Broxterman H. J., van der Hoeven J. J., Pinedo H. M., Lankelma J. Potentiation of doxorubicin cytotoxicity by the calcium antagonist bepridil in anthracycline-resistant and -sensitive cell lines. A comparison with verapamil. Cancer Chemother Pharmacol. 1987;20(4):285–290. doi: 10.1007/BF00262578. [DOI] [PubMed] [Google Scholar]
- Sehested M., Skovsgaard T., van Deurs B., Winther-Nielsen H. Increased plasma membrane traffic in daunorubicin resistant P388 leukaemic cells. Effect of daunorubicin and verapamil. Br J Cancer. 1987 Dec;56(6):747–751. doi: 10.1038/bjc.1987.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried J. M., Tritton T. R., Sartorelli A. C. Comparison of anthracycline concentrations in S180 cell lines of varying sensitivity. Eur J Cancer Clin Oncol. 1983 Aug;19(8):1133–1141. doi: 10.1016/0277-5379(83)90039-1. [DOI] [PubMed] [Google Scholar]
- Skipper H. E. On mathematical modeling of critical variables in cancer treatment (goals: better understanding of the past and better planning in the future). Bull Math Biol. 1986;48(3-4):253–278. doi: 10.1007/BF02459681. [DOI] [PubMed] [Google Scholar]
- Tarasiuk J., Frézard F., Garnier-Suillerot A., Gattegno L. Anthracycline incorporation in human lymphocytes. Kinetics of uptake and nuclear concentration. Biochim Biophys Acta. 1989 Sep 19;1013(2):109–117. doi: 10.1016/0167-4889(89)90038-4. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Richert N. D., Cornwell M. M., Tsuruo T., Hamada H., Gottesman M. M., Pastan I. H. Immunocytochemical localization of P170 at the plasma membrane of multidrug-resistant human cells. J Histochem Cytochem. 1987 Dec;35(12):1451–1456. doi: 10.1177/35.12.2890686. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]