Abstract
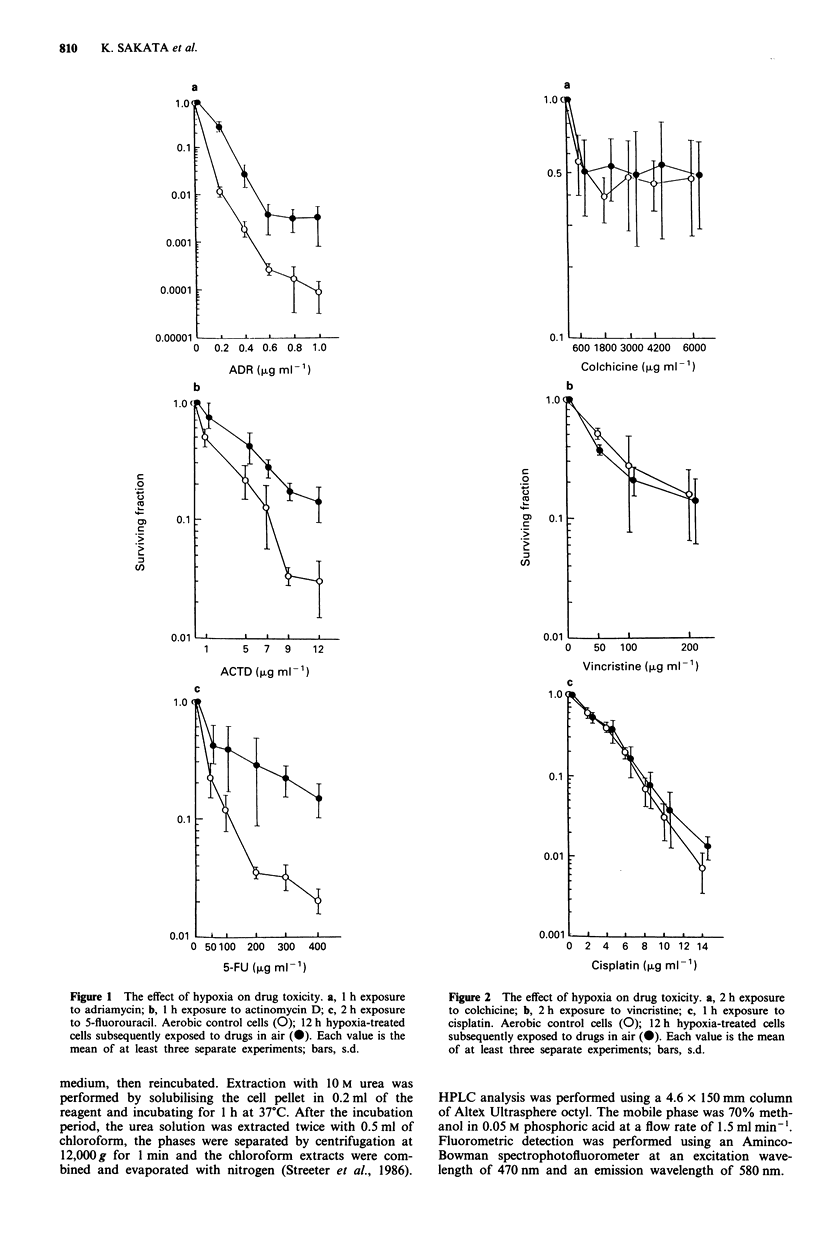
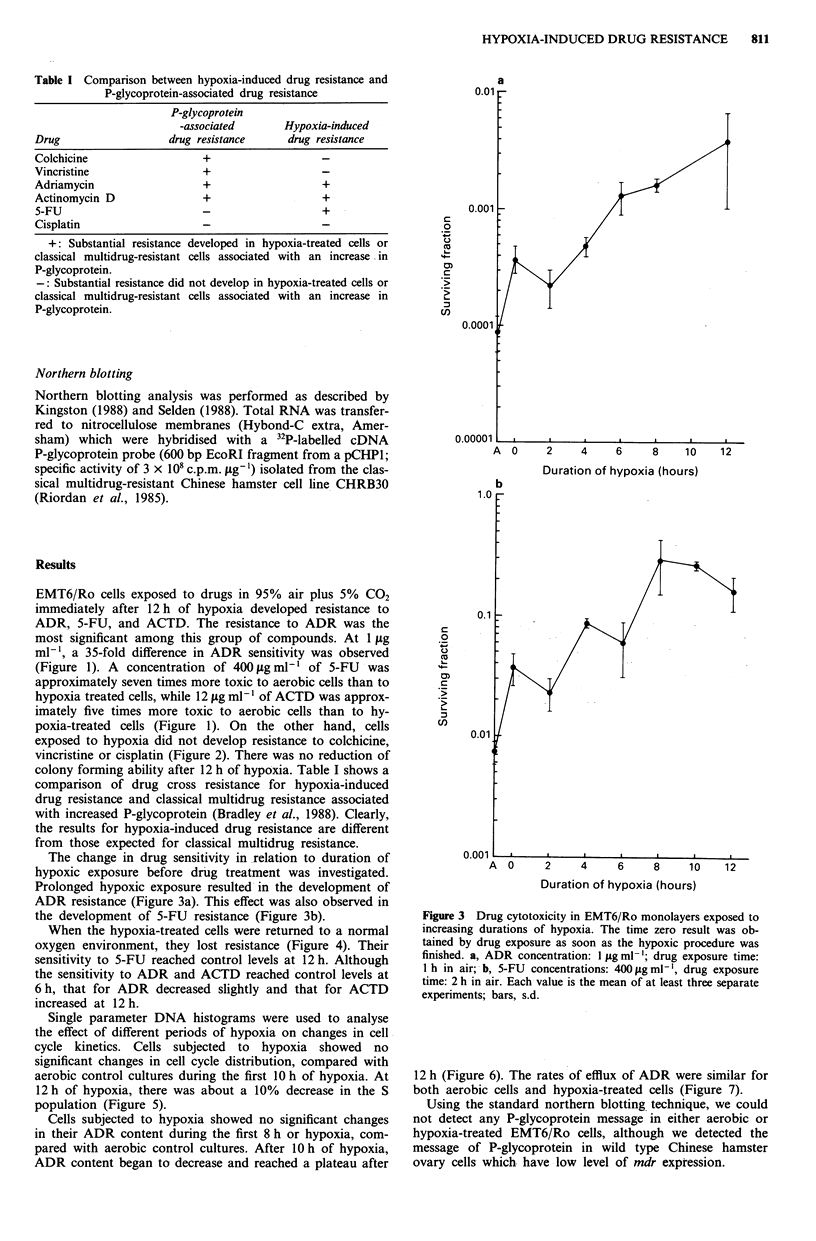
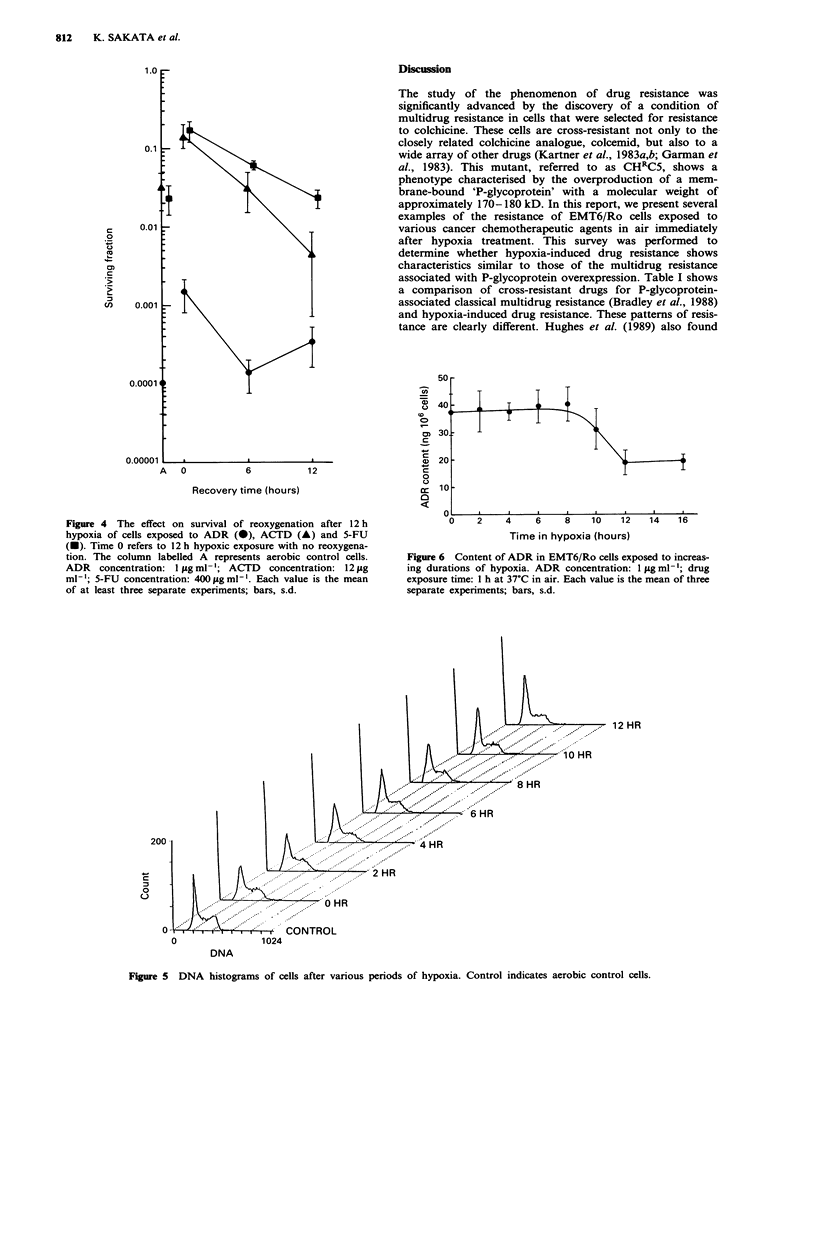
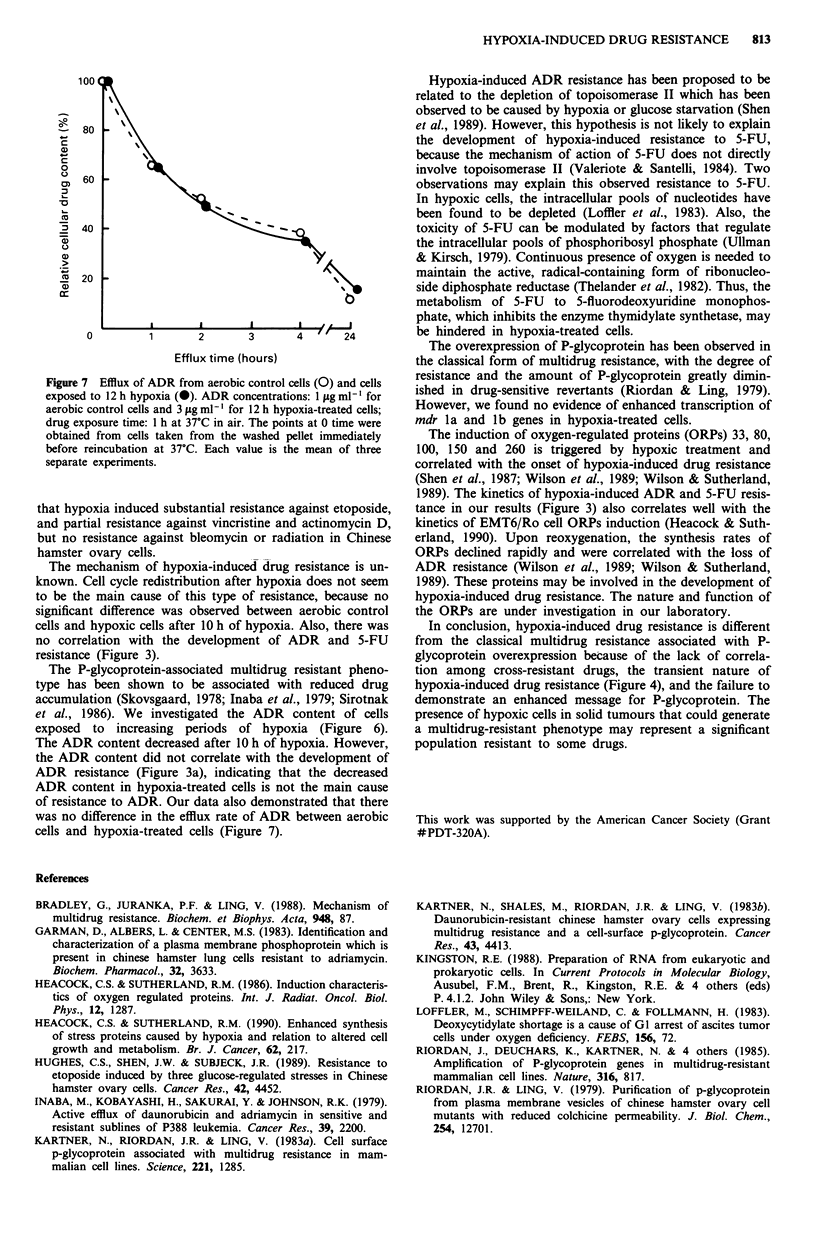
In this report, we investigate several examples of hypoxia-induced drug resistance and compare them with P-glycoprotein associated multidrug resistance (MDR). EMT6/Ro cells exposed to drugs in air immediately after hypoxic treatment developed resistance to adriamycin, 5-fluorouracil, and actinomycin D. However, these cells did not develop resistance to colchicine, vincristine or cisplatin. When the cells were returned to a normal oxygen environment, they lost resistance. There was no correlation between the content of adriamycin and the development of adriamycin resistance induced by hypoxia. There was no difference between the efflux of adriamycin from aerobic cells and that from hypoxia-treated cells. The mRNA for P-glycoprotein was not detected in the hypoxia-treated cells. These results suggest that hypoxia-induced drug resistance is different from P-glycoprotein associated multidrug resistance.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley G., Juranka P. F., Ling V. Mechanism of multidrug resistance. Biochim Biophys Acta. 1988 Aug 3;948(1):87–128. doi: 10.1016/0304-419x(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Garman D., Albers L., Center M. S. Identification and characterization of a plasma membrane phosphoprotein which is present in Chinese hamster lung cells resistant to adriamycin. Biochem Pharmacol. 1983 Dec 1;32(23):3633–3637. doi: 10.1016/0006-2952(83)90315-5. [DOI] [PubMed] [Google Scholar]
- Heacock C. S., Sutherland R. M. Enhanced synthesis of stress proteins caused by hypoxia and relation to altered cell growth and metabolism. Br J Cancer. 1990 Aug;62(2):217–225. doi: 10.1038/bjc.1990.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heacock C. S., Sutherland R. M. Induction characteristics of oxygen regulated proteins. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1287–1290. doi: 10.1016/0360-3016(86)90155-0. [DOI] [PubMed] [Google Scholar]
- Hughes C. S., Shen J. W., Subjeck J. R. Resistance to etoposide induced by three glucose-regulated stresses in Chinese hamster ovary cells. Cancer Res. 1989 Aug 15;49(16):4452–4454. [PubMed] [Google Scholar]
- Inaba M., Kobayashi H., Sakurai Y., Johnson R. K. Active efflux of daunorubicin and adriamycin in sensitive and resistant sublines of P388 leukemia. Cancer Res. 1979 Jun;39(6 Pt 1):2200–2203. [PubMed] [Google Scholar]
- Kartner N., Riordan J. R., Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983 Sep 23;221(4617):1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- Kartner N., Shales M., Riordan J. R., Ling V. Daunorubicin-resistant Chinese hamster ovary cells expressing multidrug resistance and a cell-surface P-glycoprotein. Cancer Res. 1983 Sep;43(9):4413–4419. [PubMed] [Google Scholar]
- Löffler M., Schimpff-Weiland G., Follmann H. Deoxycytidylate shortage is a cause of G1 arrest of ascites tumor cells under oxygen deficiency. FEBS Lett. 1983 May 30;156(1):72–76. doi: 10.1016/0014-5793(83)80251-8. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Deuchars K., Kartner N., Alon N., Trent J., Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. 1985 Aug 29-Sep 4Nature. 316(6031):817–819. doi: 10.1038/316817a0. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Ling V. Purification of P-glycoprotein from plasma membrane vesicles of Chinese hamster ovary cell mutants with reduced colchicine permeability. J Biol Chem. 1979 Dec 25;254(24):12701–12705. [PubMed] [Google Scholar]
- Shen J. W., Subjeck J. R., Lock R. B., Ross W. E. Depletion of topoisomerase II in isolated nuclei during a glucose-regulated stress response. Mol Cell Biol. 1989 Aug;9(8):3284–3291. doi: 10.1128/mcb.9.8.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Hughes C., Chao C., Cai J., Bartels C., Gessner T., Subjeck J. Coinduction of glucose-regulated proteins and doxorubicin resistance in Chinese hamster cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3278–3282. doi: 10.1073/pnas.84.10.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotnak F. M., Yang C. H., Mines L. S., Oribé E., Biedler J. L. Markedly altered membrane transport and intracellular binding of vincristine in multidrug-resistant Chinese hamster cells selected for resistance to vinca alkaloids. J Cell Physiol. 1986 Feb;126(2):266–274. doi: 10.1002/jcp.1041260217. [DOI] [PubMed] [Google Scholar]
- Skovsgaard T. Mechanism of cross-resistance between vincristine and daunorubicin in Ehrlich ascites tumor cells. Cancer Res. 1978 Dec;38(12):4722–4727. [PubMed] [Google Scholar]
- Smith E., Stratford I. J., Adams G. E. Cytotoxicity of adriamycin on aerobic and hypoxic chinese hamster V79 cells in vitro. Br J Cancer. 1980 Oct;42(4):568–573. doi: 10.1038/bjc.1980.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter D. G., Johl J. S., Gordon G. R., Peters J. H. Uptake and retention of morpholinyl anthracyclines by adriamycin-sensitive and -resistant P388 cells. Cancer Chemother Pharmacol. 1986;16(3):247–252. doi: 10.1007/BF00293986. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988 Apr 8;240(4849):177–184. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M., Eddy H. A., Bareham B., Reich K., Vanantwerp D. Resistance to adriamycin in multicellular spheroids. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1225–1230. doi: 10.1016/0360-3016(79)90643-6. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M., Keng P., Conroy P. J., McDermott D., Bareham B. J., Passalacqua W. In vitro hypoxic cytotoxicity of nitroimidazoles: uptake and cell cycle phase specificity. Int J Radiat Oncol Biol Phys. 1982 Mar-Apr;8(3-4):745–748. doi: 10.1016/0360-3016(82)90726-x. [DOI] [PubMed] [Google Scholar]
- Teicher B. A., Holden S. A., Rose C. M. Effect of oxygen on the cytotoxicity and antitumor activity of etoposide. J Natl Cancer Inst. 1985 Dec;75(6):1129–1133. [PubMed] [Google Scholar]
- Teicher B. A., Lazo J. S., Sartorelli A. C. Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res. 1981 Jan;41(1):73–81. [PubMed] [Google Scholar]
- Thelander L., Gräslund A., Thelander M. Continual presence of oxygen and iron required for mammalian ribonucleotide reduction: possible regulation mechanism. Biochem Biophys Res Commun. 1983 Feb 10;110(3):859–865. doi: 10.1016/0006-291x(83)91040-9. [DOI] [PubMed] [Google Scholar]
- Ullman B., Kirsch J. Metabolism of 5-fluorouracil in cultured cells. Protection from 5-fluorouracil cytotoxicity by purines. Mol Pharmacol. 1979 Mar;15(2):357–366. [PubMed] [Google Scholar]
- Valeriote F., Santelli G. 5-Fluorouracil (FUra). Pharmacol Ther. 1984;24(1):107–132. doi: 10.1016/0163-7258(84)90030-5. [DOI] [PubMed] [Google Scholar]
- Wilson R. E., Keng P. C., Sutherland R. M. Drug resistance in Chinese hamster ovary cells during recovery from severe hypoxia. J Natl Cancer Inst. 1989 Aug 16;81(16):1235–1240. doi: 10.1093/jnci/81.16.1235. [DOI] [PubMed] [Google Scholar]
- Wilson R. E., Sutherland R. M. Enhanced synthesis of specific proteins, RNA, and DNA caused by hypoxia and reoxygenation. Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):957–961. doi: 10.1016/0360-3016(89)90895-x. [DOI] [PubMed] [Google Scholar]


