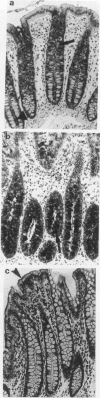
Abstract
Small intestine mucin antigen (SIMA) is an oncofoetal antigen for the colon and is distinct from the normal large intestinal mucin antigen (LIMA). In the present study, a panel of anti-SIMA and anti-LIMA monoclonal antibodies (MAb) was used to charaterise altered mucin expression in colorectal adenocarcinomas, by immunohistochemistry and quantitative immunoassays of tissue extracts. These results are compared with CEA expression and correlated with various clinicopathological indices. All mucin MAb reacted with a high proportion of the 100 colon cancers of every stage, histological type (including non-mucinous cancers), differentiation, site, or size. Inappropriate SIMA production was detected by either anti-SIMA MAb 4D3 or 4A1, even in 85% of early stage cancers. MAb 4D3 reacted with a higher proportion of cancers of smaller size and better differentiation. At the subcellular level, both anti-SIMA MAb showed reactivity typical of normal mucin, i.e., goblet cell and extracellular mucin. The normal colonic antigen, LIMA, was also detectable in the majority of cases, but quantitatively overproduced in some cases and reduced in others. However, in contrast to SIMA, LIMA was detected in predominantly undifferentiated cancer cells but not in goblet cells. Heterogeneity of MAb reactivity between cases and complementarity within each cancer was frequently observed. Mucin reactive with at least one of the MAb was detected in all of the CEA-negative cancers. A high rate of inappropriate SIMA expression was also detected in the perineoplastic transitional mucosa (88%, c.f. CEA, 35%) and adjacent, morphologically normal mucosa (80% c.f. CEA, 24%), indicating biochemical changes similar to the cancer. This panel of anti-mucin MAb demonstrated altered mucin glycoprotein metabolism associated with the development and progression of most colorectal cancers, which emphasises their utility as indicators of neoplastic change in the colon, and their superiority to CEA.
Full text
PDF

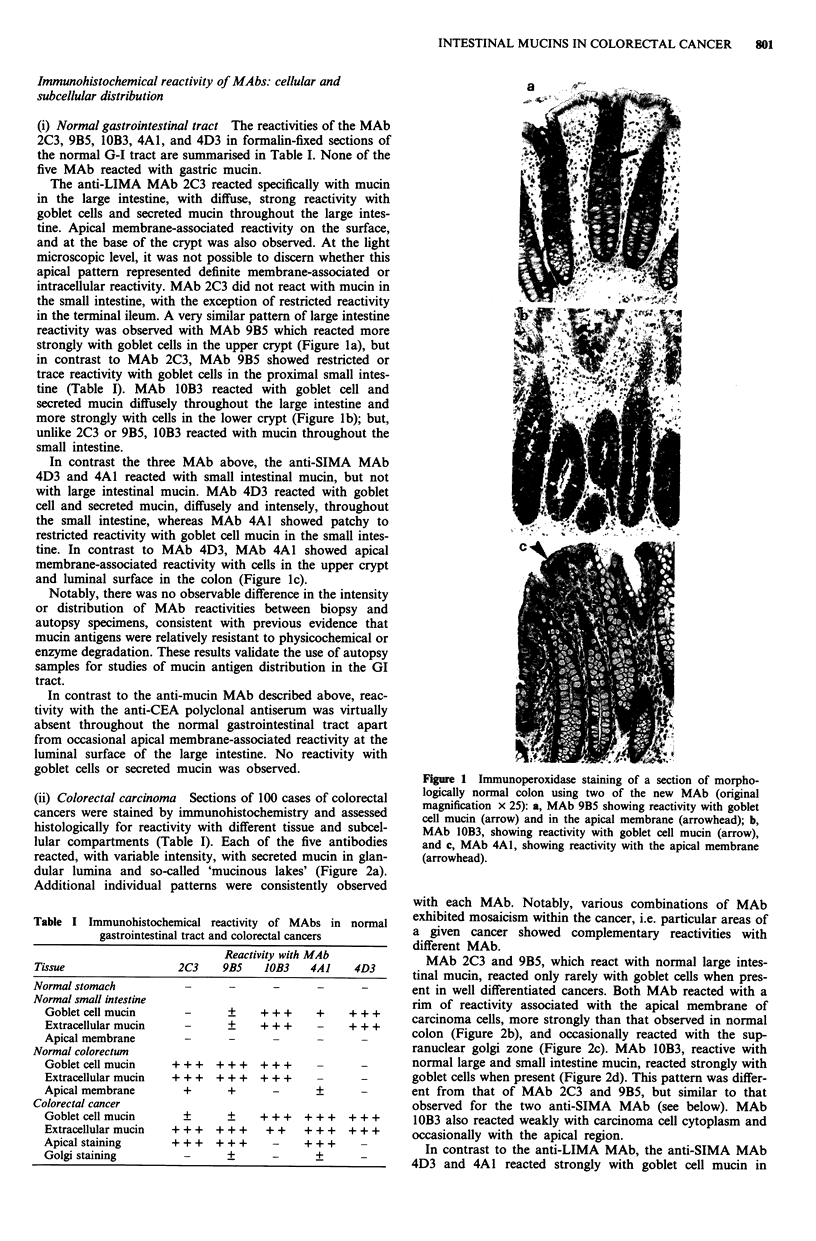
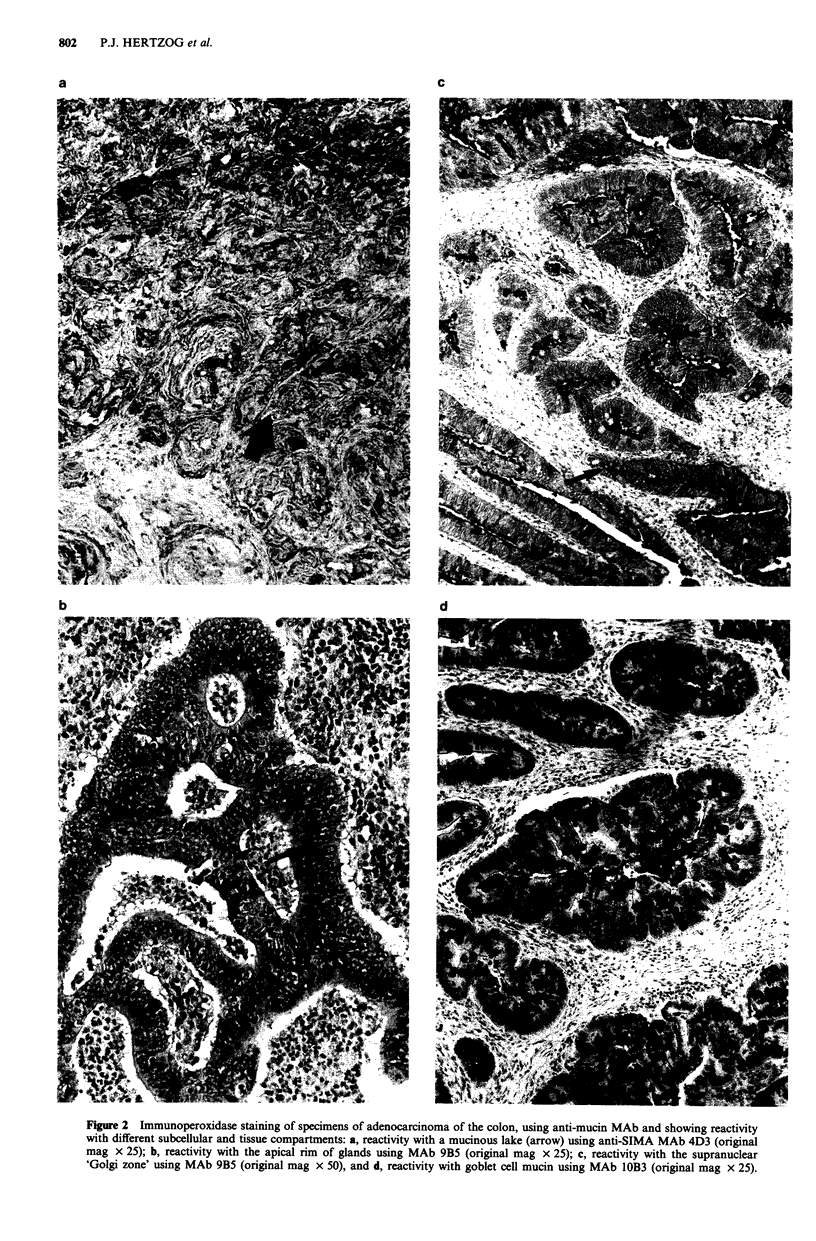

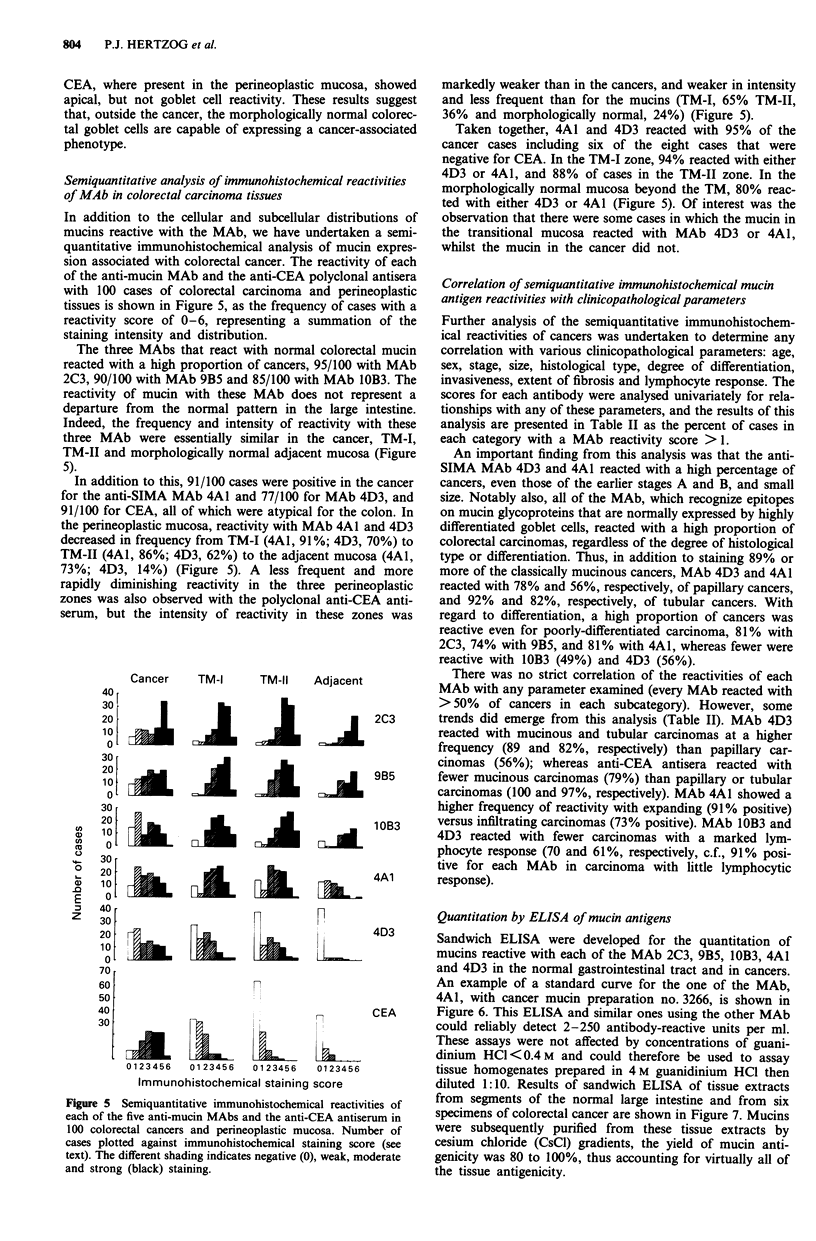
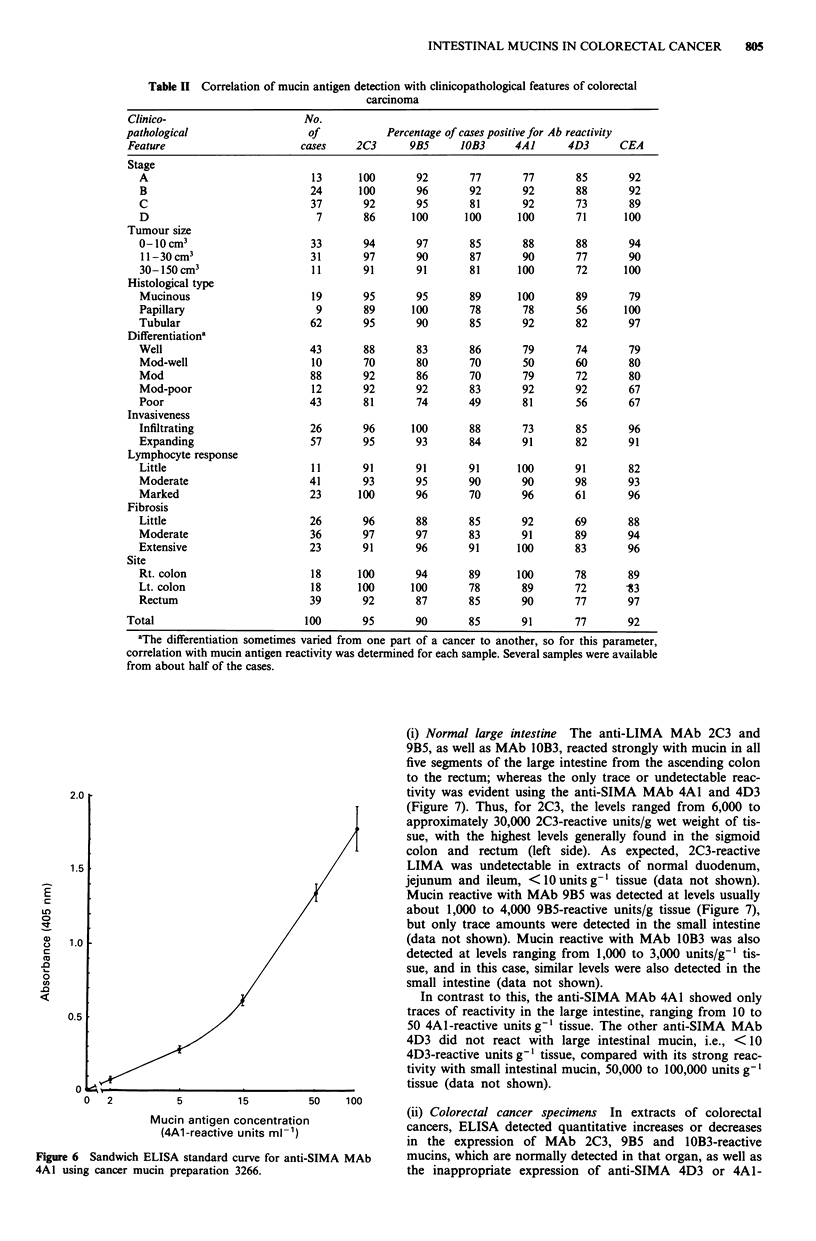
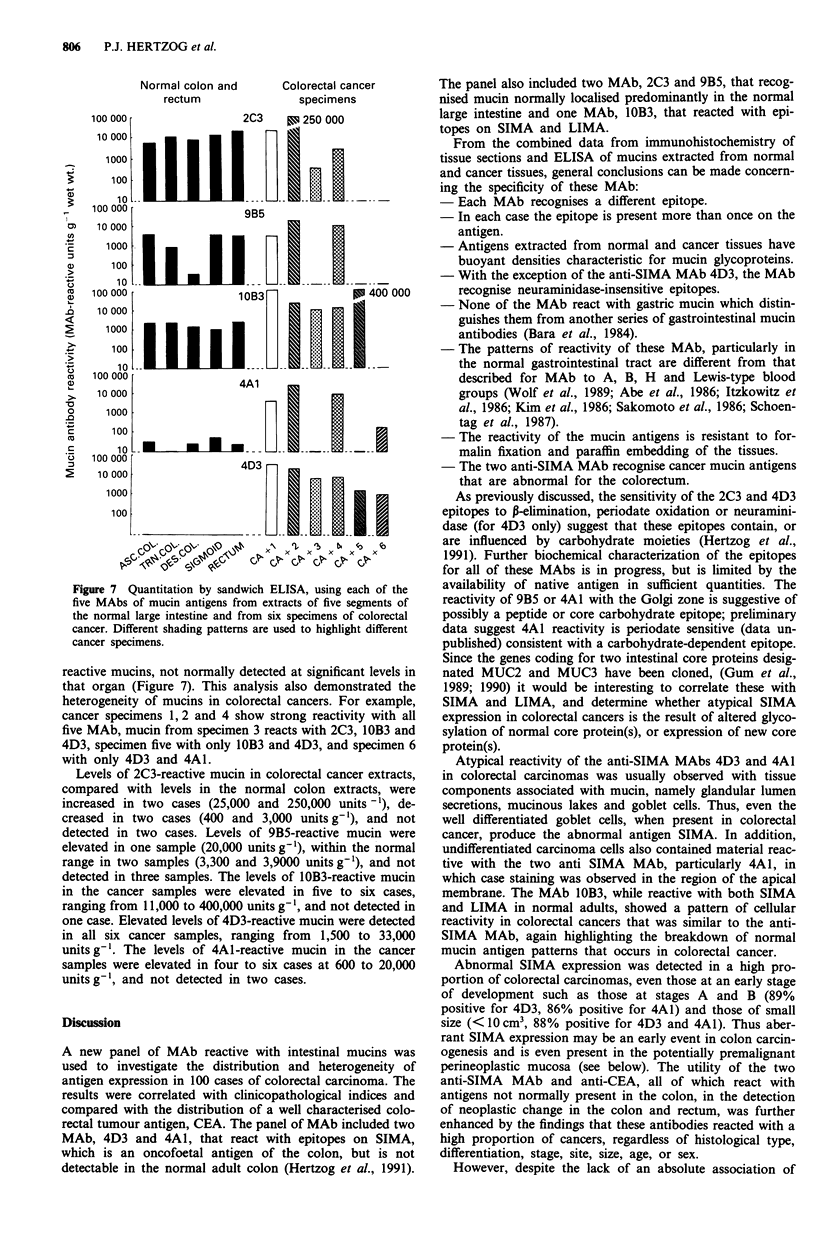


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Hakomori S., Ohshiba S. Differential expression of difucosyl type 2 chain (LeY) defined by monoclonal antibody AH6 in different locations of colonic epithelia, various histological types of colonic polyps, and adenocarcinomas. Cancer Res. 1986 May;46(5):2639–2644. [PubMed] [Google Scholar]
- Bara J., Loisillier F., Burtin P. Antigens of gastric and intestinal mucous cells in human colonic tumours. Br J Cancer. 1980 Feb;41(2):209–221. doi: 10.1038/bjc.1980.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bara J., Nardelli J., Gadenne C., Prade M., Burtin P. Differences in the expression of mucus-associated antigens between proximal and distal human colon adenocarcinomas. Br J Cancer. 1984 Apr;49(4):495–501. doi: 10.1038/bjc.1984.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M., Chatterjee S. K., Barlow J. J., Fuji H. Monoclonal antibodies recognizing tumor-associated antigen of human ovarian mucinous cystadenocarcinomas. Cancer Res. 1982 May;42(5):1650–1654. [PubMed] [Google Scholar]
- Boland C. R., Kim Y. S. Transitional mucosa of the colon and tumor growth factors. Med Hypotheses. 1987 Mar;22(3):237–243. doi: 10.1016/0306-9877(87)90189-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dawson P. A., Filipe M. I. An ultrastructural application of silver methenamine to the study of mucin changes in the colonic mucosa adjacent to and remote from carcinoma. Histochem J. 1976 Mar;8(2):143–158. doi: 10.1007/BF01007165. [DOI] [PubMed] [Google Scholar]
- Decaens C., Bara J., Rosa B., Daher N., Burtin P. Early oncofetal antigenic modifications during rat colonic carcinogenesis. Cancer Res. 1983 Jan;43(1):355–362. [PubMed] [Google Scholar]
- Filipe M. I. Value of histochemical reactions for mucosubstances in the diagnosis of certain pathological conditions of the colon and rectum. Gut. 1969 Jul;10(7):577–586. doi: 10.1136/gut.10.7.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold D. V., Miller F. Comparison of human colonic mucoprotein antigen from normal and neoplastic mucosa. Cancer Res. 1978 Oct;38(10):3204–3211. [PubMed] [Google Scholar]
- Greaves P., Filipe M. I., Branfoot A. C. Transitional mucosa and survival in human colorectal cancer. Cancer. 1980 Aug 15;46(4):764–770. doi: 10.1002/1097-0142(19800815)46:4<764::aid-cncr2820460421>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Griffiths A. B., Burchell J., Gendler S., Lewis A., Blight K., Tilly R., Taylor-Papadimitriou J. Immunological analysis of mucin molecules expressed by normal and malignant mammary epithelial cells. Int J Cancer. 1987 Sep 15;40(3):319–327. doi: 10.1002/ijc.2910400307. [DOI] [PubMed] [Google Scholar]
- Gum J. R., Byrd J. C., Hicks J. W., Toribara N. W., Lamport D. T., Kim Y. S. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989 Apr 15;264(11):6480–6487. [PubMed] [Google Scholar]
- Gum J. R., Hicks J. W., Swallow D. M., Lagace R. L., Byrd J. C., Lamport D. T., Siddiki B., Kim Y. S. Molecular cloning of cDNAs derived from a novel human intestinal mucin gene. Biochem Biophys Res Commun. 1990 Aug 31;171(1):407–415. doi: 10.1016/0006-291x(90)91408-k. [DOI] [PubMed] [Google Scholar]
- Hakomori S., Kannagi R. Glycosphingolipids as tumor-associated and differentiation markers. J Natl Cancer Inst. 1983 Aug;71(2):231–251. [PubMed] [Google Scholar]
- Hamada Y., Yamamura M., Hioki K., Yamamoto M., Nagura H., Watanabe K. Immunohistochemical study of carcinoembryonic antigen in patients with colorectal cancer. Correlation with plasma carcinoembryonic antigen levels. Cancer. 1985 Jan 1;55(1):136–141. doi: 10.1002/1097-0142(19850101)55:1<136::aid-cncr2820550121>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hertzog P. J., Robinson H. C., Ma J., Mackay I. R., Linnane A. W. Oncofetal expression of the human intestinal mucin glycoprotein antigens in gastrointestinal epithelium defined by monoclonal antibodies. Int J Cancer. 1991 May 30;48(3):355–363. doi: 10.1002/ijc.2910480308. [DOI] [PubMed] [Google Scholar]
- Isaacson P., Attwood P. R. Failure to demonstrate specificity of the morphological and histochemical changes in mucosa adjacent to colonic carcinoma (transitional mucosa). J Clin Pathol. 1979 Mar;32(3):214–218. doi: 10.1136/jcp.32.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzkowitz S. H., Yuan M., Fukushi Y., Palekar A., Phelps P. C., Shamsuddin A. M., Trump B. F., Hakomori S., Kim Y. S. Lewisx- and sialylated Lewisx-related antigen expression in human malignant and nonmalignant colonic tissues. Cancer Res. 1986 May;46(5):2627–2632. [PubMed] [Google Scholar]
- Jass J. R., Atkin W. S., Cuzick J., Bussey H. J., Morson B. C., Northover J. M., Todd I. P. The grading of rectal cancer: historical perspectives and a multivariate analysis of 447 cases. Histopathology. 1986 May;10(5):437–459. doi: 10.1111/j.1365-2559.1986.tb02497.x. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Yuan M., Itzkowitz S. H., Sun Q. B., Kaizu T., Palekar A., Trump B. F., Hakomori S. Expression of LeY and extended LeY blood group-related antigens in human malignant, premalignant, and nonmalignant colonic tissues. Cancer Res. 1986 Nov;46(11):5985–5992. [PubMed] [Google Scholar]
- Ma J., de Boer W. G., Ward H. A., Nairn R. C. Another oncofoetal antigen in colonic carcinoma. Br J Cancer. 1980 Feb;41(2):325–328. doi: 10.1038/bjc.1980.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasaki H., Mitomi T., Noto T., Ogoshi K., Hanaue H., Tanaka Y., Makuuchi H., Clausen H., Hakomori S. Mosaicism in the expression of tumor-associated carbohydrate antigens in human colonic and gastric cancers. Cancer Res. 1989 Jul 1;49(13):3662–3669. [PubMed] [Google Scholar]
- Sakamoto J., Furukawa K., Cordon-Cardo C., Yin B. W., Rettig W. J., Oettgen H. F., Old L. J., Lloyd K. O. Expression of Lewisa, Lewisb, X, and Y blood group antigens in human colonic tumors and normal tissue and in human tumor-derived cell lines. Cancer Res. 1986 Mar;46(3):1553–1561. [PubMed] [Google Scholar]
- Schoentag R., Primus F. J., Kuhns W. ABH and Lewis blood group expression in colorectal carcinoma. Cancer Res. 1987 Mar 15;47(6):1695–1700. [PubMed] [Google Scholar]
- Wolf B. C., Salem R. R., Sears H. F., Horst D. A., Lavin P. T., Herlyn M., Itzkowitz S. H., Schlom J., Steele G. D., Jr The expression of colorectal carcinoma-associated antigens in the normal colonic mucosa. An immunohistochemical analysis of regional distribution. Am J Pathol. 1989 Jul;135(1):111–119. [PMC free article] [PubMed] [Google Scholar]