Abstract
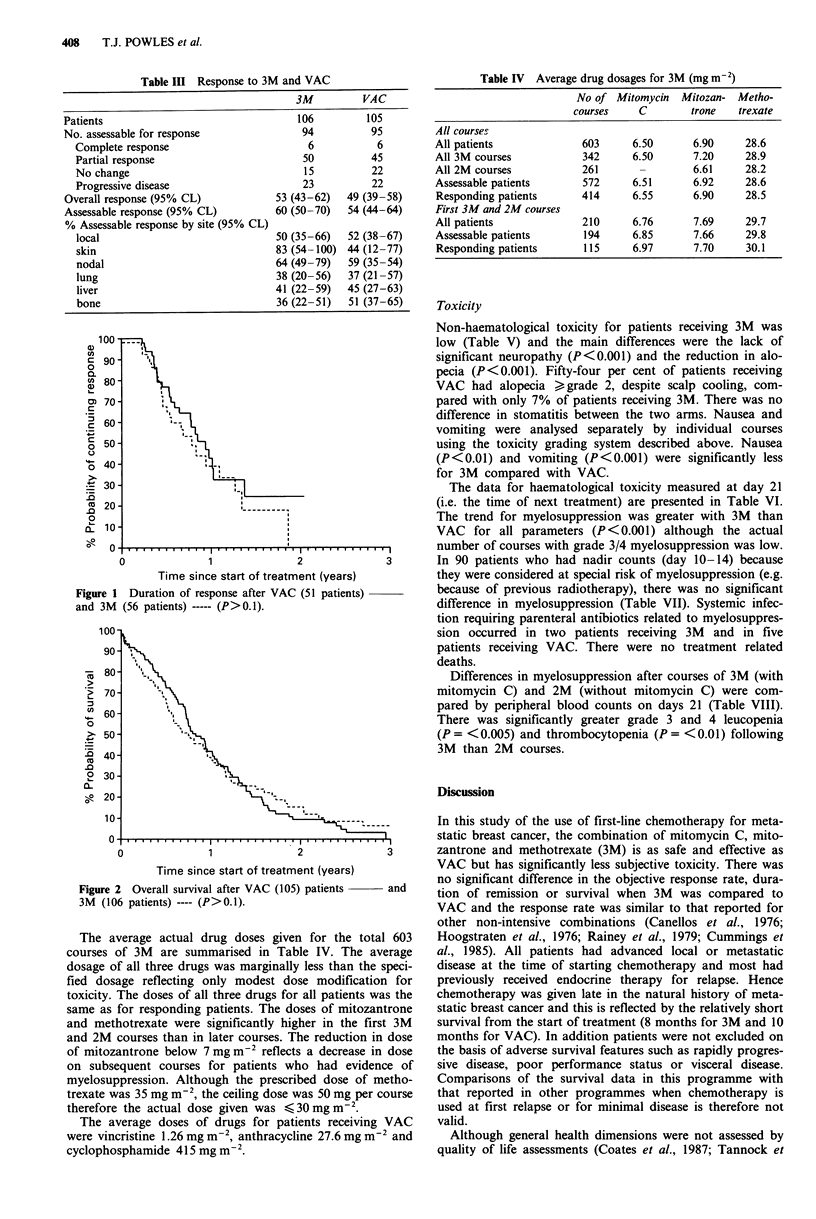
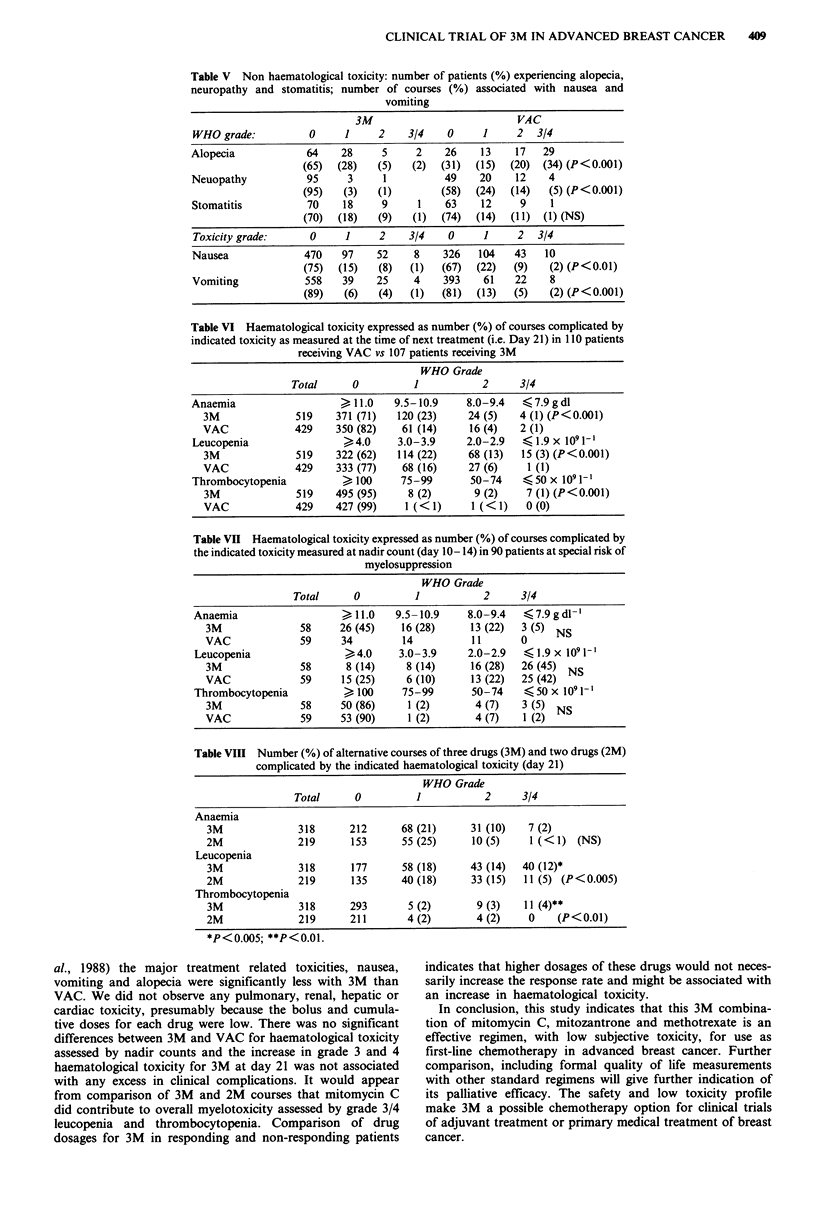
This paper describes a randomised clinical trial in patients with advanced breast cancer, comparing the regimen 3M, mitomycin C 7-8 mg m-2 (day 1), mitozantrone 7-8 mg m-2 (day 1 and 21), methotrexate 35 mg m-2 (day 1 and 21) given on a 42 day cycle with a standard anthracycline containing regimen, VAC, vincristine 1.4 mg m-2 (day 1), anthracycline (adriamycin or epirubicin) 30 mg m-2 (day 1), cyclophosphamide 400 mg m-2 (day 1) given on a 21 day cycle. Of a total of 217 patients, 107 were randomised to 3M and 110 to VAC and a mean of 5.5 courses was given per patient. The overall response rate (complete and partial) was 53% (95% Confidence Limits (CL): 43-62%) for 3M and 49% (CL; 39-58%) for VAC. The response according to sites of metastases was the same for both treatment groups. Symptomatic toxicity including alopecia, neuropathy, vomiting (P less than 0.001) and nausea (P less than 0.01) were significantly less for 3M. Myelosuppression including leucopenia (P less than 0.001) and thrombocytopenia (P less than 0.001) was significantly greater with 3M at day 21, although there was no difference in nadir counts in patients at special risk of myelosuppression and there was no evidence of an increase in infective or bleeding complications. There was no significant difference in the duration of response to 3M (10 months, CL 6-15) and VAC (11 months, CL 7-12), nor in survival (3M, 8 months, CL 6-12; VAC, 10 months, CL 8-12). These results indicate that 3M is as effective as, but has significantly less symptomatic toxicity than, an anthracycline containing regimen for the treatment of advanced breast cancer.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canellos G. P., DeVita V. T., Gold G. L., Chabner B. A., Schein P. S., Young R. C. Combination chemotherapy for advanced breast cancer: response and effect on survival. Ann Intern Med. 1976 Apr;84(4):389–392. doi: 10.7326/0003-4819-84-4-389. [DOI] [PubMed] [Google Scholar]
- Carbone P. P., Bauer M., Band P., Tormey D. Chemotherapy of disseminated breast cancer. Current status and prospects. Cancer. 1977 Jun;39(6 Suppl):2916–2922. doi: 10.1002/1097-0142(197706)39:6<2916::aid-cncr2820390678>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Carter S. K. Integration of chemotherapy into combined modality treatment of solid tumors VII. Adenocarcinoma of the breast. Cancer Treat Rev. 1976 Sep;3(3):141–174. doi: 10.1016/s0305-7372(76)80020-5. [DOI] [PubMed] [Google Scholar]
- Coates A., Gebski V., Bishop J. F., Jeal P. N., Woods R. L., Snyder R., Tattersall M. H., Byrne M., Harvey V., Gill G. Improving the quality of life during chemotherapy for advanced breast cancer. A comparison of intermittent and continuous treatment strategies. N Engl J Med. 1987 Dec 10;317(24):1490–1495. doi: 10.1056/NEJM198712103172402. [DOI] [PubMed] [Google Scholar]
- Coombes R. C., Powles T. J., Gazet J. C., Nash A. G., Ford H. T., McKinna A., Neville A. M. Assessment of biochemical tests to screen for metastases in patients with breast cancer. Lancet. 1980 Feb 9;1(8163):296–297. doi: 10.1016/s0140-6736(80)90790-4. [DOI] [PubMed] [Google Scholar]
- Cornbleet M. A., Stuart-Harris R. C., Smith I. E., Coleman R. E., Rubens R. D., McDonald M., Mouridsen H. T., Rainer H., van Oosterom A. T., Smyth J. F. Mitoxantrone for the treatment of advanced breast cancer: single-agent therapy in previously untreated patients. Eur J Cancer Clin Oncol. 1984 Sep;20(9):1141–1146. doi: 10.1016/0277-5379(84)90122-6. [DOI] [PubMed] [Google Scholar]
- Cummings F. J., Gelman R., Horton J. Comparison of CAF versus CMFP in metastatic breast cancer: analysis of prognostic factors. J Clin Oncol. 1985 Jul;3(7):932–940. doi: 10.1200/JCO.1985.3.7.932. [DOI] [PubMed] [Google Scholar]
- Eder J. P., Antman K., Peters W., Henner W. D., Elias A., Shea T., Schryber S., Andersen J., Come S., Schnipper L. High-dose combination alkylating agent chemotherapy with autologous bone marrow support for metastatic breast cancer. J Clin Oncol. 1986 Nov;4(11):1592–1597. doi: 10.1200/JCO.1986.4.11.1592. [DOI] [PubMed] [Google Scholar]
- Hayward J. L., Carbone P. P., Heusen J. C., Kumaoka S., Segaloff A., Rubens R. D. Assessment of response to therapy in advanced breast cancer. Br J Cancer. 1977 Mar;35(3):292–298. doi: 10.1038/bjc.1977.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. B., Holland J. F., Bhardwaj S., Norton L., Wilfinger C., Strashun A. A phase I-II study of intensive-dose adriamycin for advanced breast cancer. J Clin Oncol. 1987 Feb;5(2):172–177. doi: 10.1200/JCO.1987.5.2.172. [DOI] [PubMed] [Google Scholar]
- Perez D. J., Powles T. J., Gazet J. C., Ford H. T., Coombes R. C. Mitomycin C, melphalan and methotrexate combination chemotherapy for palliation of disseminated breast cancer. Cancer Chemother Pharmacol. 1984;13(1):36–38. doi: 10.1007/BF00401444. [DOI] [PubMed] [Google Scholar]
- Powles T. J., Coombes R. C., Smith I. E., Jones J. M., Ford H. T., Gazet J. C. Failure of chemotherapy to prolong survival in a group of patients with metastatic breast cancer. Lancet. 1980 Mar 15;1(8168 Pt 1):580–582. doi: 10.1016/s0140-6736(80)91066-1. [DOI] [PubMed] [Google Scholar]
- Rainey J. M., Jones S. E., Salmon S. E. Combination chemotherapy for advanced breast cancer utilizing vincristine, adriamycin, and cyclophosphamide (VAC). Cancer. 1979 Jan;43(1):66–71. doi: 10.1002/1097-0142(197901)43:1<66::aid-cncr2820430109>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Rosner D., Nemoto T., Lane W. W. A randomized study of intensive versus moderate chemotherapy programs in metastatic breast cancer. Cancer. 1987 Mar 1;59(5):874–883. doi: 10.1002/1097-0142(19870301)59:5<874::aid-cncr2820590503>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Tannock I. F., Boyd N. F., DeBoer G., Erlichman C., Fine S., Larocque G., Mayers C., Perrault D., Sutherland H. A randomized trial of two dose levels of cyclophosphamide, methotrexate, and fluorouracil chemotherapy for patients with metastatic breast cancer. J Clin Oncol. 1988 Sep;6(9):1377–1387. doi: 10.1200/JCO.1988.6.9.1377. [DOI] [PubMed] [Google Scholar]


