Abstract
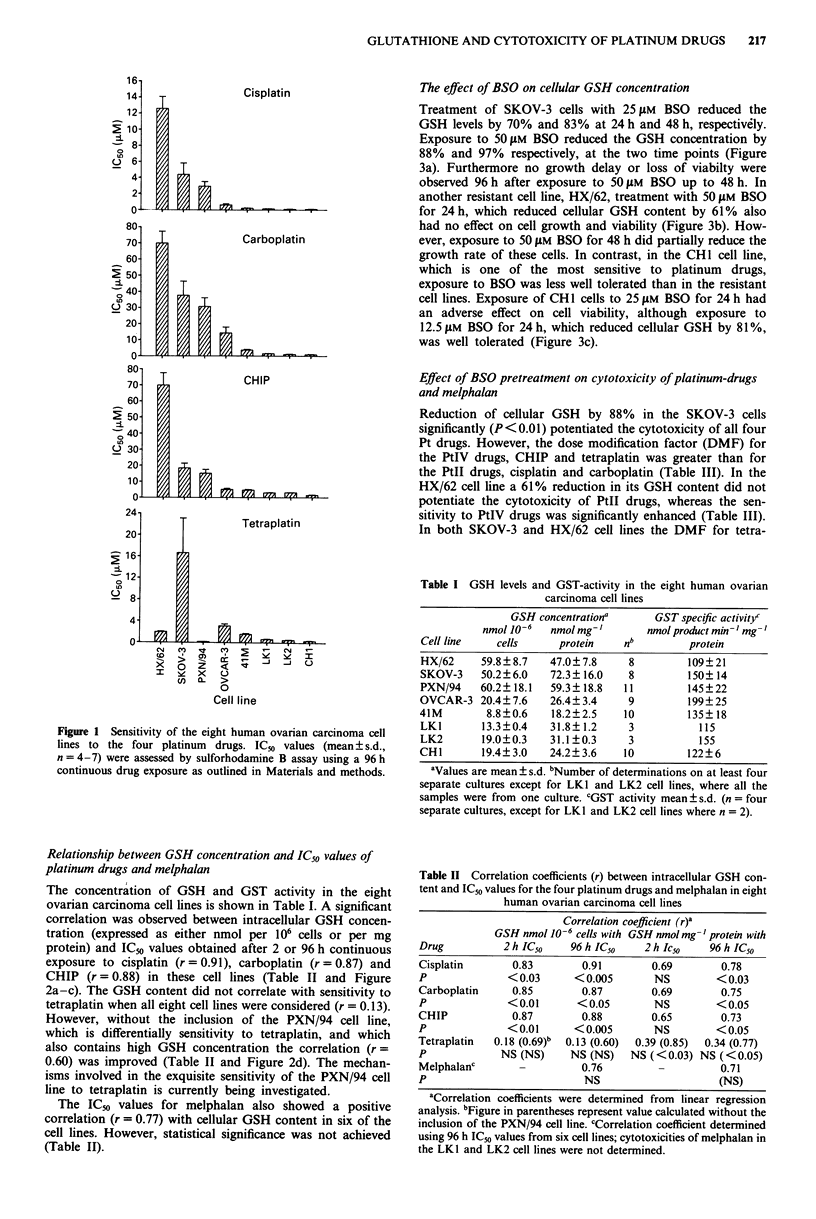
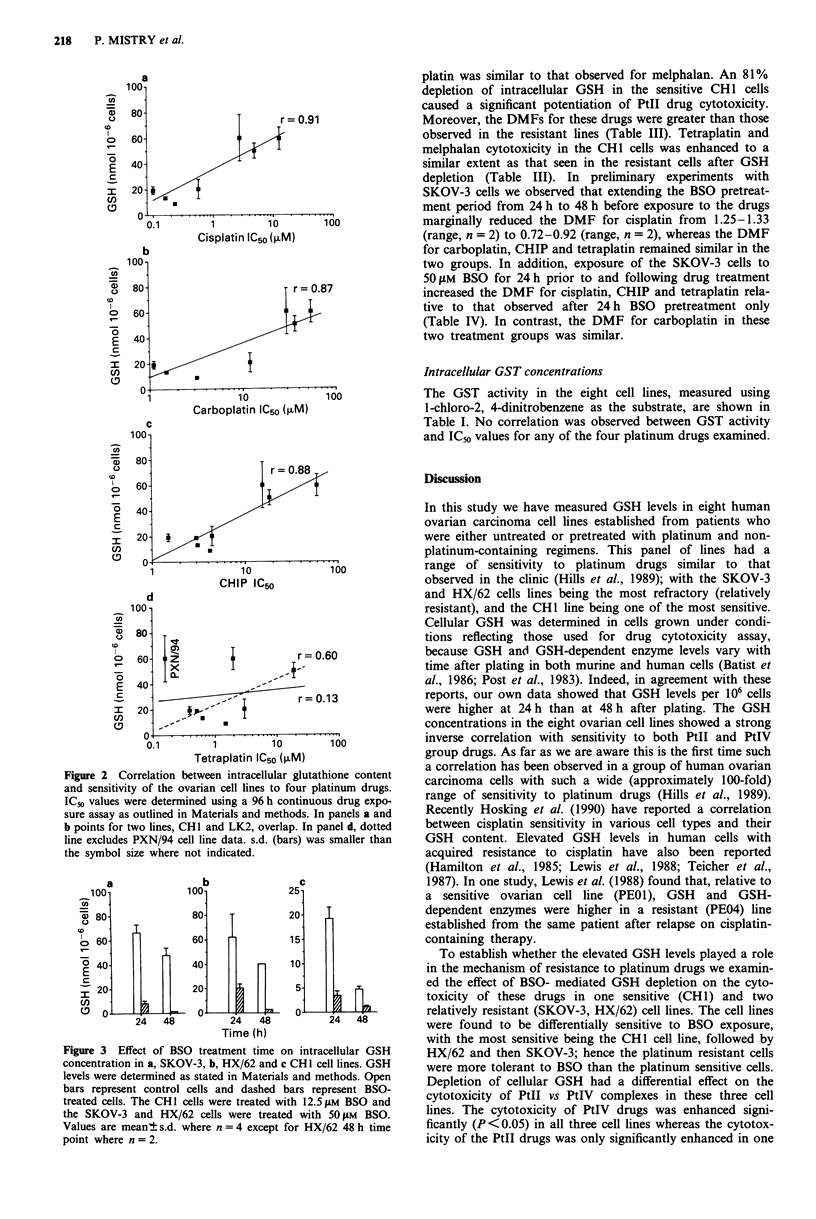
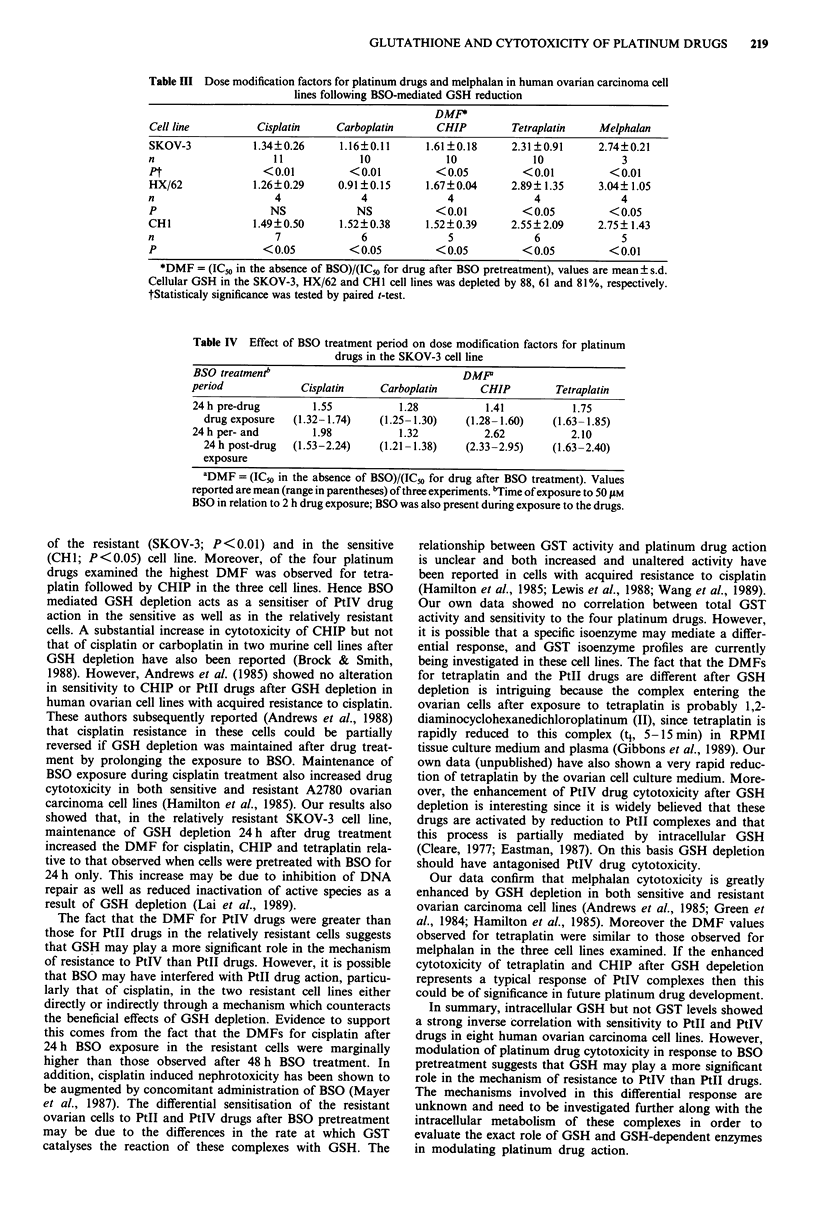
The role of glutathione (GSH) and GSH-S-transferase (GST) activity in modulating the cytotoxicity of four platinum drugs and melphalan was evaluated in eight human ovarian carcinoma cell lines. The cell lines were established from solid and ascitic tumours from pretreated and untreated patients, and showed a wide spectrum of sensitivity to several platinum II and platinum IV drugs; cisplatin, carboplatin, CHIP and tetraplatin. Intracellular glutathione concentration measured in the cell lines showed a significant (P = 0.05) correlation with IC50 values for cisplatin (r = 0.91), carboplatin (r = 0.87) and CHIP (r = 0.88). The correlation between GSH levels and IC50 values for melphalan (r = 0.76) or tetraplatin (r = 0.60) was not as significant. GST activity showed no correlation with IC50 values, for the four platinum drugs. To determine the significance of the elevated GSH concentration in the refractory cell lines, the effect of D,L-buthionine-S, R-sulfoximine (BSO) mediated GSH depletion on platinum drug cytotoxicity was examined in one of the most sensitive (CH1) and two of the least sensitive (relatively resistant; SKOV-3, HX/62) cell lines. Comparison was made with the effect of GSH depletion on melphalan cytotoxicity in these three lines. These lines were differentially sensitive to BSO, with the two most platinum drug resistant lines being more tolerant to BSO than the sensitive CH1 line. Depletion of cellular GSH, ranging between 61 and 88%, had a differential effect on the sensitivity to PtII vs PtIV drugs in the three cell lines: cytotoxicity of the PtIV drugs, tetraplatin and CHIP, was substantially enhanced in both the resistant and sensitive cell lines; in contrast, the cytotoxicity of the PtII drugs, cisplatin and carboplatin, was only significantly increased in one of the two relatively resistant lines (SKOV-3) and in the sensitive (CH1) line after GSH depletion. Moreover the dose modification factor (DMF) for the PtII agents were lower than those for PtIV agents in the three cell lines. The dose modification factor for tetraplatin after BSO treatment was similar to that observed for melphalan in all three cell lines. In the SKOV-3 cell line extending the BSO pretreatment period to 48 h from 24 h marginally reduced the cytotoxicity of cisplatin, whereas the cytotoxicity of the other three drugs remained similar to that observed after 24 h BSO pretreatment. In contrast, extending the BSO treatment to 24 h after drug exposure potentiated the cytotoxicity of cisplatin, CHIP and tetraplatin.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. A., Howell S. B. Cellular pharmacology of cisplatin: perspectives on mechanisms of acquired resistance. Cancer Cells. 1990 Feb;2(2):35–43. [PubMed] [Google Scholar]
- Andrews P. A., Murphy M. P., Howell S. B. Differential potentiation of alkylating and platinating agent cytotoxicity in human ovarian carcinoma cells by glutathione depletion. Cancer Res. 1985 Dec;45(12 Pt 1):6250–6253. [PubMed] [Google Scholar]
- Andrews P. A., Schiefer M. A., Murphy M. P., Howell S. B. Enhanced potentiation of cisplatin cytotoxicity in human ovarian carcinoma cells by prolonged glutathione depletion. Chem Biol Interact. 1988;65(1):51–58. doi: 10.1016/0009-2797(88)90030-0. [DOI] [PubMed] [Google Scholar]
- Arrick B. A., Nathan C. F. Glutathione metabolism as a determinant of therapeutic efficacy: a review. Cancer Res. 1984 Oct;44(10):4224–4232. [PubMed] [Google Scholar]
- Batist G., Behrens B. C., Makuch R., Hamilton T. C., Katki A. G., Louie K. G., Myers C. E., Ozols R. F. Serial determinations of glutathione levels and glutathione-related enzyme activities in human tumor cells in vitro. Biochem Pharmacol. 1986 Jul 1;35(13):2257–2259. doi: 10.1016/0006-2952(86)90601-5. [DOI] [PubMed] [Google Scholar]
- Calvert A. H., Harland S. J., Newell D. R., Siddik Z. H., Harrap K. R. Phase I studies with carboplatin at the Royal Marsden Hospital. Cancer Treat Rev. 1985 Sep;12 (Suppl A):51–57. doi: 10.1016/0305-7372(85)90018-0. [DOI] [PubMed] [Google Scholar]
- Eastman A. Glutathione-mediated activation of anticancer platinum(IV) complexes. Biochem Pharmacol. 1987 Dec 1;36(23):4177–4178. doi: 10.1016/0006-2952(87)90581-8. [DOI] [PubMed] [Google Scholar]
- Gibbons G. R., Wyrick S., Chaney S. G. Rapid reduction of tetrachloro(D,L-trans)1,2-diaminocyclohexaneplatinum(IV) (tetraplatin) in RPMI 1640 tissue culture medium. Cancer Res. 1989 Mar 15;49(6):1402–1407. [PubMed] [Google Scholar]
- Green J. A., Vistica D. T., Young R. C., Hamilton T. C., Rogan A. M., Ozols R. F. Potentiation of melphalan cytotoxicity in human ovarian cancer cell lines by glutathione depletion. Cancer Res. 1984 Nov;44(11):5427–5431. [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hamilton T. C., Lai G. M., Rothenberg M. L., Fojo A. T., Young R. C., Ozols R. F. Mechanisms of resistance to cisplatin and alkylating agents. Cancer Treat Res. 1989;48:151–169. doi: 10.1007/978-1-4613-1601-5_10. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Winker M. A., Louie K. G., Batist G., Behrens B. C., Tsuruo T., Grotzinger K. R., McKoy W. M., Young R. C., Ozols R. F. Augmentation of adriamycin, melphalan, and cisplatin cytotoxicity in drug-resistant and -sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochem Pharmacol. 1985 Jul 15;34(14):2583–2586. doi: 10.1016/0006-2952(85)90551-9. [DOI] [PubMed] [Google Scholar]
- Hills C. A., Kelland L. R., Abel G., Siracky J., Wilson A. P., Harrap K. R. Biological properties of ten human ovarian carcinoma cell lines: calibration in vitro against four platinum complexes. Br J Cancer. 1989 Apr;59(4):527–534. doi: 10.1038/bjc.1989.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking L. K., Whelan R. D., Shellard S. A., Bedford P., Hill B. T. An evaluation of the role of glutathione and its associated enzymes in the expression of differential sensitivities to antitumour agents shown by a range of human tumour cell lines. Biochem Pharmacol. 1990 Oct 15;40(8):1833–1842. doi: 10.1016/0006-2952(90)90364-q. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai G. M., Ozols R. F., Young R. C., Hamilton T. C. Effect of glutathione on DNA repair in cisplatin-resistant human ovarian cancer cell lines. J Natl Cancer Inst. 1989 Apr 5;81(7):535–539. doi: 10.1093/jnci/81.7.535. [DOI] [PubMed] [Google Scholar]
- Lewis A. D., Hayes J. D., Wolf C. R. Glutathione and glutathione-dependent enzymes in ovarian adenocarcinoma cell lines derived from a patient before and after the onset of drug resistance: intrinsic differences and cell cycle effects. Carcinogenesis. 1988 Jul;9(7):1283–1287. doi: 10.1093/carcin/9.7.1283. [DOI] [PubMed] [Google Scholar]
- Mayer R. D., Lee K. E., Cockett A. T. Inhibition of cisplatin-induced nephrotoxicity in rats by buthionine sulfoximine, a glutathione synthesis inhibitor. Cancer Chemother Pharmacol. 1987;20(3):207–210. doi: 10.1007/BF00570486. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Peckham M. J., Horwich A., Brada M., Drury A., Hendry W. F. cis-Diammine-1, 1-cyclobutane dicarboxylate platinum II (carboplatin) in the treatment of testicular germ-cell tumours: a preliminary report. Cancer Treat Rev. 1985 Sep;12 (Suppl A):101–110. doi: 10.1016/0305-7372(85)90025-8. [DOI] [PubMed] [Google Scholar]
- Post G. B., Keller D. A., Connor K. A., Menzel D. B. Effects of culture conditions on glutathione content in A549 cells. Biochem Biophys Res Commun. 1983 Jul 29;114(2):737–742. doi: 10.1016/0006-291x(83)90842-2. [DOI] [PubMed] [Google Scholar]
- Richon V. M., Schulte N., Eastman A. Multiple mechanisms of resistance to cis-diamminedichloroplatinum(II) in murine leukemia L1210 cells. Cancer Res. 1987 Apr 15;47(8):2056–2061. [PubMed] [Google Scholar]
- Russo A., DeGraff W., Friedman N., Mitchell J. B. Selective modulation of glutathione levels in human normal versus tumor cells and subsequent differential response to chemotherapy drugs. Cancer Res. 1986 Jun;46(6):2845–2848. [PubMed] [Google Scholar]
- Smith E., Brock A. P. An in vitro study comparing the cytotoxicity of three platinum complexes with regard to the effect of thiol depletion. Br J Cancer. 1988 Jun;57(6):548–552. doi: 10.1038/bjc.1988.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Y., Teicher B. A., Shea T. C., Holden S. A., Rosbe K. W., al-Achi A., Henner W. D. Cross-resistance and glutathione-S-transferase-pi levels among four human melanoma cell lines selected for alkylating agent resistance. Cancer Res. 1989 Nov 15;49(22):6185–6192. [PubMed] [Google Scholar]
- de Graeff A., Slebos R. J., Rodenhuis S. Resistance to cisplatin and analogues: mechanisms and potential clinical implications. Cancer Chemother Pharmacol. 1988;22(4):325–332. doi: 10.1007/BF00254240. [DOI] [PubMed] [Google Scholar]


