Abstract
The doxorubicin-selected multidrug resistant small cell lung cancer cell line, H69AR, is cross-resistant to the Vinca alkaloids and epipodophyllotoxins, but does not overexpress P-glycoprotein, a 170 kDa plasma membrane efflux pump usually associated with this type of resistance. Monoclonal antibodies were raised against the H69AR cell line and one of these, MAb 3.186, recognises a peptide epitope on a 36 kDa phosphorylated protein that is membrane associated, but not presented on the external surface of H69AR cells (Mirski & Cole, 1991). In the present study, in vitro translation and molecular cloning techniques were used to determine the relative levels of mRNA corresponding to the 3.186 antigen. In addition, a cDNA clone containing an insert of approximately 1.4 kb was obtained by screening an H69AR cDNA library with 125I-MAb 3.186. Fragments of this cloned DNA hybridised to a single mRNA species of approximately 1.6 kb that was 5 to 6-fold elevated in H69AR cells. Partial DNA sequencing and restriction endonuclease mapping revealed identity of the cloned DNA with p36, a member of the annexin/lipocortin family of Ca2+ and phospholipid binding proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas F., Jongsma A. P., Broxterman H. J., Arceci R. J., Housman D., Scheffer G. L., Riethorst A., van Groenigen M., Nieuwint A. W., Joenje H. Non-P-glycoprotein mediated mechanism for multidrug resistance precedes P-glycoprotein expression during in vitro selection for doxorubicin resistance in a human lung cancer cell line. Cancer Res. 1990 Sep 1;50(17):5392–5398. [PubMed] [Google Scholar]
- Barnes J. A., Michiel D., Hollenberg M. D. Simultaneous phosphorylation of three human calpactins by kinase C. Biochem Cell Biol. 1991 Feb-Mar;69(2-3):163–169. doi: 10.1139/o91-024. [DOI] [PubMed] [Google Scholar]
- Brugge J. S. The p35/p36 substrates of protein-tyrosine kinases as inhibitors of phospholipase A2. Cell. 1986 Jul 18;46(2):149–150. doi: 10.1016/0092-8674(86)90729-4. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. Control of exocytosis in adrenal chromaffin cells. Biochim Biophys Acta. 1991 Jul 22;1071(2):174–202. doi: 10.1016/0304-4157(91)90024-q. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Janowska-Wieczorek A., Lynch M. A., Sheinin H., Hindenburg A. A., Beck W. T. Effect of duration of exposure to verapamil on vincristine activity against multidrug-resistant human leukemic cell lines. Cancer Res. 1989 Nov 1;49(21):5798–5804. [PubMed] [Google Scholar]
- Cole S. P., Chanda E. R., Dicke F. P., Gerlach J. H., Mirski S. E. Non-P-glycoprotein-mediated multidrug resistance in a small cell lung cancer cell line: evidence for decreased susceptibility to drug-induced DNA damage and reduced levels of topoisomerase II. Cancer Res. 1991 Jul 1;51(13):3345–3352. [PubMed] [Google Scholar]
- Cole S. P. Patterns of cross-resistance in a multidrug-resistant small-cell lung carcinoma cell line. Cancer Chemother Pharmacol. 1990;26(4):250–256. doi: 10.1007/BF02897225. [DOI] [PubMed] [Google Scholar]
- Cole S. P. Rapid chemosensitivity testing of human lung tumor cells using the MTT assay. Cancer Chemother Pharmacol. 1986;17(3):259–263. doi: 10.1007/BF00256695. [DOI] [PubMed] [Google Scholar]
- Crompton M. R., Moss S. E., Crumpton M. J. Diversity in the lipocortin/calpactin family. Cell. 1988 Oct 7;55(1):1–3. doi: 10.1016/0092-8674(88)90002-5. [DOI] [PubMed] [Google Scholar]
- Crumpton M. J., Dedman J. R. Protein terminology tangle. Nature. 1990 May 17;345(6272):212–212. doi: 10.1038/345212a0. [DOI] [PubMed] [Google Scholar]
- Davidson F. F., Dennis E. A., Powell M., Glenney J. R., Jr Inhibition of phospholipase A2 by "lipocortins" and calpactins. An effect of binding to substrate phospholipids. J Biol Chem. 1987 Feb 5;262(4):1698–1705. [PubMed] [Google Scholar]
- Deuchars K. L., Ling V. P-glycoprotein and multidrug resistance in cancer chemotherapy. Semin Oncol. 1989 Apr;16(2):156–165. [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Erikson E., Tomasiewicz H. G., Erikson R. L. Biochemical characterization of a 34-kilodalton normal cellular substrate of pp60v-src and an associated 6-kilodalton protein. Mol Cell Biol. 1984 Jan;4(1):77–85. doi: 10.1128/mcb.4.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J. D., Hoye E., Blackwood R. A., Mok T. L. Identification of a domain that mediates vesicle aggregation reveals functional diversity of annexin repeats. J Biol Chem. 1991 Apr 15;266(11):6670–6673. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fox M. T., Prentice D. A., Hughes J. P. Increases in p11 and annexin II proteins correlate with differentiation in the PC12 pheochromocytoma. Biochem Biophys Res Commun. 1991 Jun 28;177(3):1188–1193. doi: 10.1016/0006-291x(91)90666-u. [DOI] [PubMed] [Google Scholar]
- Frohlich M., Motté P., Galvin K., Takahashi H., Wands J., Ozturk M. Enhanced expression of the protein kinase substrate p36 in human hepatocellular carcinoma. Mol Cell Biol. 1990 Jun;10(6):3216–3223. doi: 10.1128/mcb.10.6.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V. Tyrosine protein kinase substrate p36: a member of the annexin family of Ca2+/phospholipid-binding proteins. Cell Motil Cytoskeleton. 1989;14(4):449–454. doi: 10.1002/cm.970140402. [DOI] [PubMed] [Google Scholar]
- Gerlach J. H., Endicott J. A., Juranka P. F., Henderson G., Sarangi F., Deuchars K. L., Ling V. Homology between P-glycoprotein and a bacterial haemolysin transport protein suggests a model for multidrug resistance. Nature. 1986 Dec 4;324(6096):485–489. doi: 10.1038/324485a0. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Boudreau M., Galyean R., Hunter T., Tack B. Association of the S-100-related calpactin I light chain with the NH2-terminal tail of the 36-kDa heavy chain. J Biol Chem. 1986 Aug 15;261(23):10485–10488. [PubMed] [Google Scholar]
- Glenney J. R., Jr, Tack B. F. Amino-terminal sequence of p36 and associated p10: identification of the site of tyrosine phosphorylation and homology with S-100. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7884–7888. doi: 10.1073/pnas.82.23.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Tack B., Powell M. A. Calpactins: two distinct Ca++-regulated phospholipid- and actin-binding proteins isolated from lung and placenta. J Cell Biol. 1987 Mar;104(3):503–511. doi: 10.1083/jcb.104.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Woodgett J. R., Isacke C. M., Hunter T. The protein-tyrosine kinase substrate p36 is also a substrate for protein kinase C in vitro and in vivo. Mol Cell Biol. 1986 Jul;6(7):2738–2744. doi: 10.1128/mcb.6.7.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. S., Wallner B. P., Mattaliano R. J., Tizard R., Burne C., Frey A., Hession C., McGray P., Sinclair L. K., Chow E. P. Two human 35 kd inhibitors of phospholipase A2 are related to substrates of pp60v-src and of the epidermal growth factor receptor/kinase. Cell. 1986 Jul 18;46(2):191–199. doi: 10.1016/0092-8674(86)90736-1. [DOI] [PubMed] [Google Scholar]
- Isacke C. M., Lindberg R. A., Hunter T. Synthesis of p36 and p35 is increased when U-937 cells differentiate in culture but expression is not inducible by glucocorticoids. Mol Cell Biol. 1989 Jan;9(1):232–240. doi: 10.1128/mcb.9.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal H. K., Chaney W. G., Anderson C. W., Davis R. G., Vishwanatha J. K. The protein-tyrosine kinase substrate, calpactin I heavy chain (p36), is part of the primer recognition protein complex that interacts with DNA polymerase alpha. J Biol Chem. 1991 Mar 15;266(8):5169–5176. [PubMed] [Google Scholar]
- Johnsson N., Marriott G., Weber K. p36, the major cytoplasmic substrate of src tyrosine protein kinase, binds to its p11 regulatory subunit via a short amino-terminal amphiphatic helix. EMBO J. 1988 Aug;7(8):2435–2442. doi: 10.1002/j.1460-2075.1988.tb03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S. L., Goldstein L. J., Gottesman M. M., Pastan I., Tsai C. M., Johnson B. E., Mulshine J. L., Ihde D. C., Kayser K., Gazdar A. F. MDR1 gene expression in lung cancer. J Natl Cancer Inst. 1989 Aug 2;81(15):1144–1150. doi: 10.1093/jnci/81.15.1144. [DOI] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
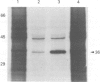
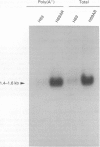
- Mirski S. E., Cole S. P. Antigens associated with multidrug resistance in H69AR, a small cell lung cancer cell line. Cancer Res. 1989 Oct 15;49(20):5719–5724. [PubMed] [Google Scholar]
- Mirski S. E., Cole S. P. Multidrug resistance-associated antigens on drug-sensitive and -resistant human tumour cell lines. Br J Cancer. 1991 Jul;64(1):15–22. doi: 10.1038/bjc.1991.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirski S. E., Gerlach J. H., Cole S. P. Multidrug resistance in a human small cell lung cancer cell line selected in adriamycin. Cancer Res. 1987 May 15;47(10):2594–2598. [PubMed] [Google Scholar]
- Noonan K. E., Beck C., Holzmayer T. A., Chin J. E., Wunder J. S., Andrulis I. L., Gazdar A. F., Willman C. L., Griffith B., Von Hoff D. D. Quantitative analysis of MDR1 (multidrug resistance) gene expression in human tumors by polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7160–7164. doi: 10.1073/pnas.87.18.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Tizard R., Mattaliano R. J., Sinclair L. K., Miller G. T., Browning J. L., Chow E. P., Burne C., Huang K. S., Pratt D. Five distinct calcium and phospholipid binding proteins share homology with lipocortin I. J Biol Chem. 1988 Aug 5;263(22):10799–10811. [PubMed] [Google Scholar]
- Reeve J. G., Rabbitts P. H., Twentyman P. R. Non-P-glycoprotein-mediated multidrug resistance with reduced EGF receptor expression in a human large cell lung cancer cell line. Br J Cancer. 1990 Jun;61(6):851–855. doi: 10.1038/bjc.1990.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. R., Deuchars K., Kartner N., Alon N., Trent J., Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. 1985 Aug 29-Sep 4Nature. 316(6031):817–819. doi: 10.1038/316817a0. [DOI] [PubMed] [Google Scholar]
- Rothhut B., Comera C., Prieur B., Errasfa M., Minassian G., Russo-Marie F. Purification and characterization of a 32-kDa phospholipase A2 inhibitory protein (lipocortin) from human peripheral blood mononuclear cells. FEBS Lett. 1987 Jul 13;219(1):169–175. doi: 10.1016/0014-5793(87)81211-5. [DOI] [PubMed] [Google Scholar]
- Russo-Marie F. Lipocortins: an update. Prostaglandins Leukot Essent Fatty Acids. 1991 Feb;42(2):83–89. doi: 10.1016/0952-3278(91)90072-d. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saris C. J., Tack B. F., Kristensen T., Glenney J. R., Jr, Hunter T. The cDNA sequence for the protein-tyrosine kinase substrate p36 (calpactin I heavy chain) reveals a multidomain protein with internal repeats. Cell. 1986 Jul 18;46(2):201–212. doi: 10.1016/0092-8674(86)90737-3. [DOI] [PubMed] [Google Scholar]
- Slapak C. A., Daniel J. C., Levy S. B. Sequential emergence of distinct resistance phenotypes in murine erythroleukemia cells under adriamycin selection: decreased anthracycline uptake precedes increased P-glycoprotein expression. Cancer Res. 1990 Dec 15;50(24):7895–7901. [PubMed] [Google Scholar]
- Slovak M. L., Hoeltge G. A., Dalton W. S., Trent J. M. Pharmacological and biological evidence for differing mechanisms of doxorubicin resistance in two human tumor cell lines. Cancer Res. 1988 May 15;48(10):2793–2797. [PubMed] [Google Scholar]
- Ueyama H., Hamada H., Battula N., Kakunaga T. Structure of a human smooth muscle actin gene (aortic type) with a unique intron site. Mol Cell Biol. 1984 Jun;4(6):1073–1078. doi: 10.1128/mcb.4.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zokas L., Glenney J. R., Jr The calpactin light chain is tightly linked to the cytoskeletal form of calpactin I: studies using monoclonal antibodies to calpactin subunits. J Cell Biol. 1987 Nov;105(5):2111–2121. doi: 10.1083/jcb.105.5.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong S., Zijlstra J. G., de Vries E. G., Mulder N. H. Reduced DNA topoisomerase II activity and drug-induced DNA cleavage activity in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res. 1990 Jan 15;50(2):304–309. [PubMed] [Google Scholar]