Abstract
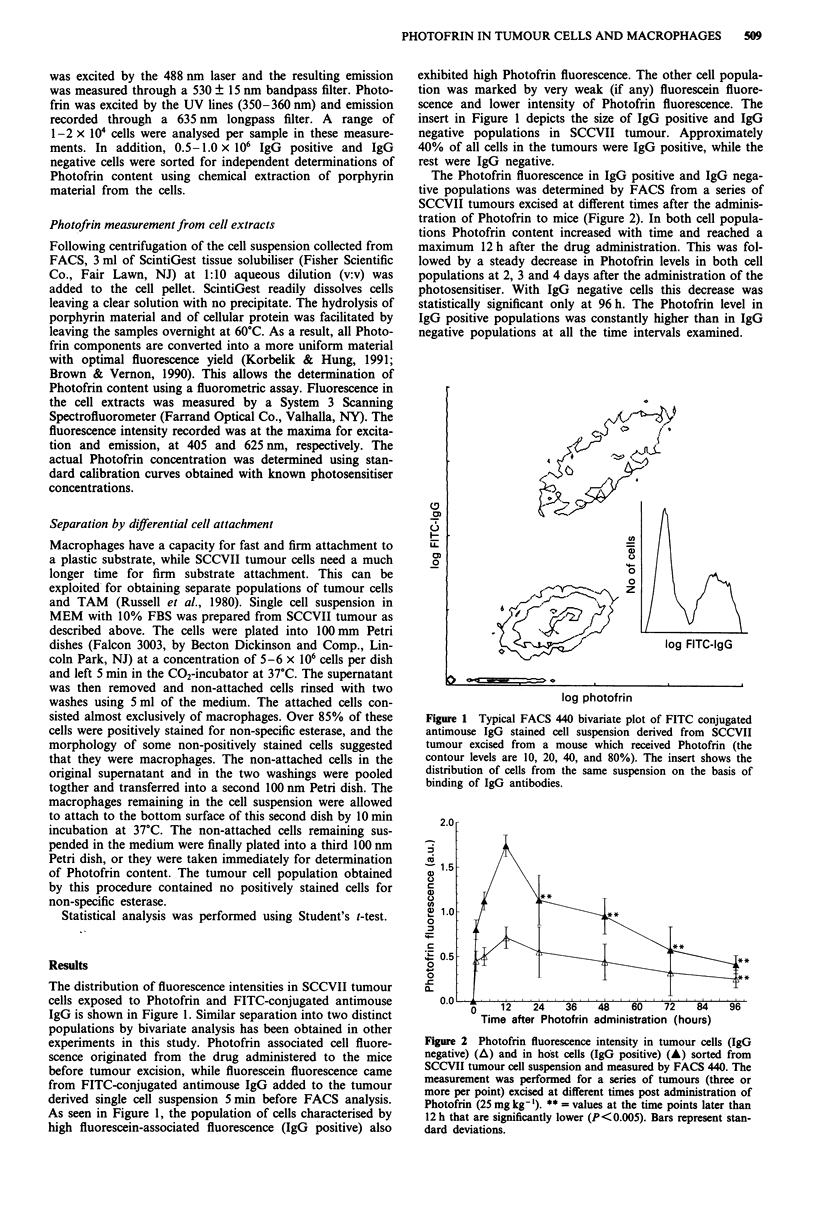
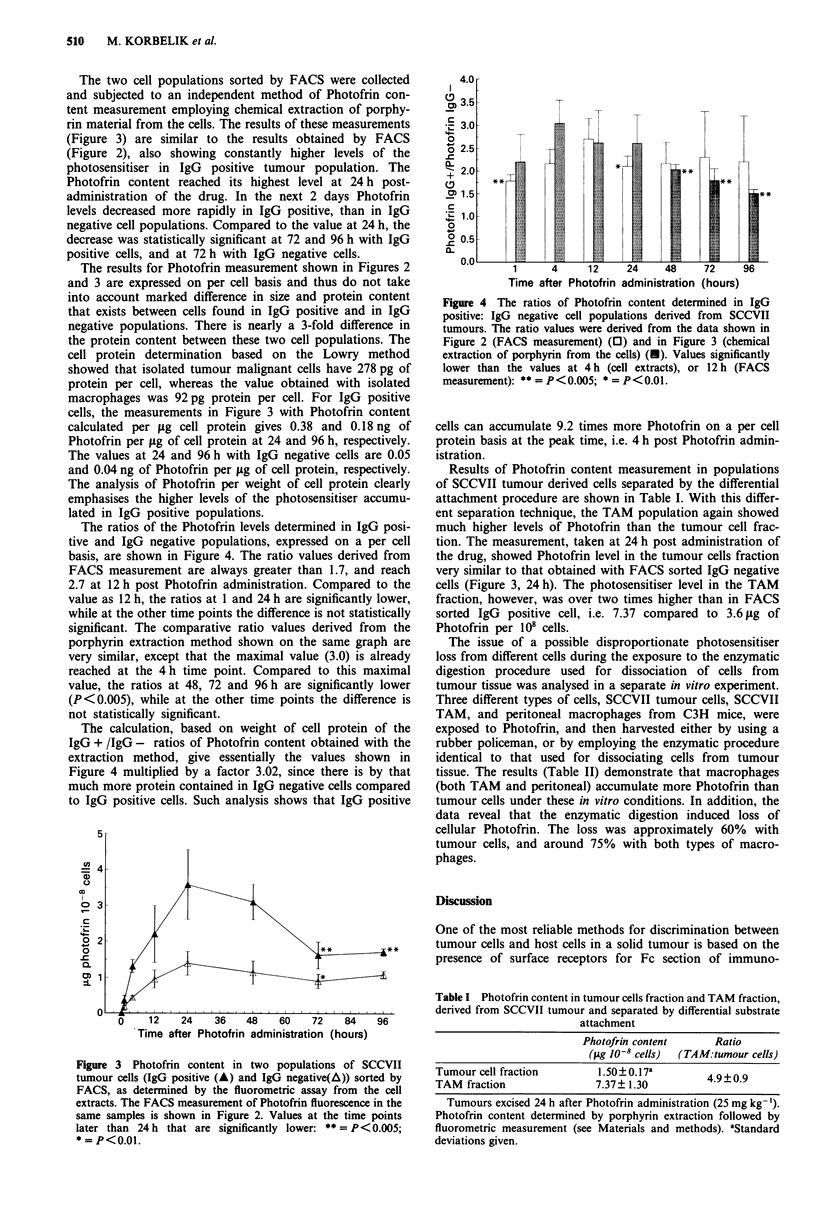
Photofrin levels in cells derived from SCCVII tumours, excised from mice that previously received the drug, were measured using a fluorescence activated cell sorter (FACS). Concomitantly, in the same cells the FACS was used to measure fluorescein isothiocyanate (FITC) fluorescence that originated from FITC-conjugated antimouse IgG added to the cell suspension before sorting. This later measurement enabled discrimination between IgG negative tumour malignant cells and IgG positive host cells (primarily macrophages). In addition, cellular Photofrin content in 'tumour' and 'host' cells sorted by FACS was determined by chemical extraction. The measurements were performed for the time intervals 1-96 h post Photofrin administration. The data showed consistently higher Photofrin levels in the 'host cells', i.e., tumour associated macrophages (TAM), than in 'tumour' cells. On a per cell basis, at any time point studied there was a minimum of 1.7 times more Photofrin in 'host' than in 'tumour cells', while at 4-12 h postadministration, ratios of up to 3.0 times were observed. This corresponds to ratio values greater than 9, when based on Photofrin content per micrograms cell protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bugelski P. J., Porter C. W., Dougherty T. J. Autoradiographic distribution of hematoporphyrin derivative in normal and tumor tissue of the mouse. Cancer Res. 1981 Nov;41(11 Pt 1):4606–4612. [PubMed] [Google Scholar]
- Chan W. S., Marshall J. F., Lam G. Y., Hart I. R. Tissue uptake, distribution, and potency of the photoactivatable dye chloroaluminum sulfonated phthalocyanine in mice bearing transplantable tumors. Cancer Res. 1988 Jun 1;48(11):3040–3044. [PubMed] [Google Scholar]
- Chaplin D. J., Olive P. L., Durand R. E. Intermittent blood flow in a murine tumor: radiobiological effects. Cancer Res. 1987 Jan 15;47(2):597–601. [PubMed] [Google Scholar]
- Darling G., Fraker D. L., Jensen J. C., Gorschboth C. M., Norton J. A. Cachectic effects of recombinant human tumor necrosis factor in rats. Cancer Res. 1990 Jul 1;50(13):4008–4013. [PubMed] [Google Scholar]
- Eccles S. A., Alexander P. Macrophage content of tumours in relation to metastatic spread and host immune reaction. Nature. 1974 Aug 23;250(5468):667–669. doi: 10.1038/250667a0. [DOI] [PubMed] [Google Scholar]
- Evans S., Matthews W., Perry R., Fraker D., Norton J., Pass H. I. Effect of photodynamic therapy on tumor necrosis factor production by murine macrophages. J Natl Cancer Inst. 1990 Jan 3;82(1):34–39. doi: 10.1093/jnci/82.1.34. [DOI] [PubMed] [Google Scholar]
- Ferrario A., Gomer C. J. Systemic toxicity in mice induced by localized porphyrin photodynamic therapy. Cancer Res. 1990 Feb 1;50(3):539–543. [PubMed] [Google Scholar]
- Henderson B. W., Bellnier D. A. Tissue localization of photosensitizers and the mechanism of photodynamic tissue destruction. Ciba Found Symp. 1989;146:112–130. doi: 10.1002/9780470513842.ch8. [DOI] [PubMed] [Google Scholar]
- Henderson B. W., Donovan J. M. Release of prostaglandin E2 from cells by photodynamic treatment in vitro. Cancer Res. 1989 Dec 15;49(24 Pt 1):6896–6900. [PubMed] [Google Scholar]
- Jori G. In vivo transport and pharmacokinetic behavior of tumour photosensitizers. Ciba Found Symp. 1989;146:78–94. doi: 10.1002/9780470513842.ch6. [DOI] [PubMed] [Google Scholar]
- Korbelik M., Hung J. Cellular delivery and retention of Photofrin II: the effects of interaction with human plasma proteins. Photochem Photobiol. 1991 Apr;53(4):501–510. doi: 10.1111/j.1751-1097.1991.tb03662.x. [DOI] [PubMed] [Google Scholar]
- Korbelik M., Krosl G., Chaplin D. J. Photofrin uptake by murine macrophages. Cancer Res. 1991 May 1;51(9):2251–2255. [PubMed] [Google Scholar]
- Lindsay J. M., Manning L., Wood G. W. Exclusive binding of immunoglobulin to Fc gamma receptors on macrophages in 3-methylcholanthrene-induced murine tumors. J Natl Cancer Inst. 1982 Nov;69(5):1163–1174. [PubMed] [Google Scholar]
- Lynch D. H., Haddad S., King V. J., Ott M. J., Straight R. C., Jolles C. J. Systemic immunosuppression induced by photodynamic therapy (PDT) is adoptively transferred by macrophages. Photochem Photobiol. 1989 Apr;49(4):453–458. doi: 10.1111/j.1751-1097.1989.tb09194.x. [DOI] [PubMed] [Google Scholar]
- Milas L., Wike J., Hunter N., Volpe J., Basic I. Macrophage content of murine sarcomas and carcinomas: associations with tumor growth parameters and tumor radiocurability. Cancer Res. 1987 Feb 15;47(4):1069–1075. [PubMed] [Google Scholar]
- Moan J., Sommer S. Uptake of the components of hematoporphyrin derivative by cells and tumours. Cancer Lett. 1983 Dec;21(2):167–174. doi: 10.1016/0304-3835(83)90204-5. [DOI] [PubMed] [Google Scholar]
- Olive P. L. Distribution, oxygenation, and clonogenicity of macrophages in a murine tumor. Cancer Commun. 1989;1(2):93–100. doi: 10.3727/095535489820875273. [DOI] [PubMed] [Google Scholar]
- Russell S. W., Gillespie G. Y., Pace J. L. Evidence for mononuclear phagocytes in solid neoplasms and appraisal of their nonspecific cytotoxic capabilities. Contemp Top Immunobiol. 1980;10:143–166. doi: 10.1007/978-1-4684-3677-8_6. [DOI] [PubMed] [Google Scholar]
- Svennevig J. L., Svaar H. Content and distribution of macrophages and lymphocytes in solid malignant human tumours. Int J Cancer. 1979 Dec 15;24(6):754–758. doi: 10.1002/ijc.2910240609. [DOI] [PubMed] [Google Scholar]
- Wood G. W., Gollahon K. A. Detection and quantitation of macrophage infiltration into primary human tumors with the use of cell-surface markers. J Natl Cancer Inst. 1977 Oct;59(4):1081–1087. doi: 10.1093/jnci/59.4.1081. [DOI] [PubMed] [Google Scholar]


