Abstract
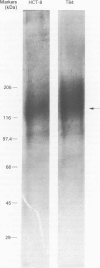
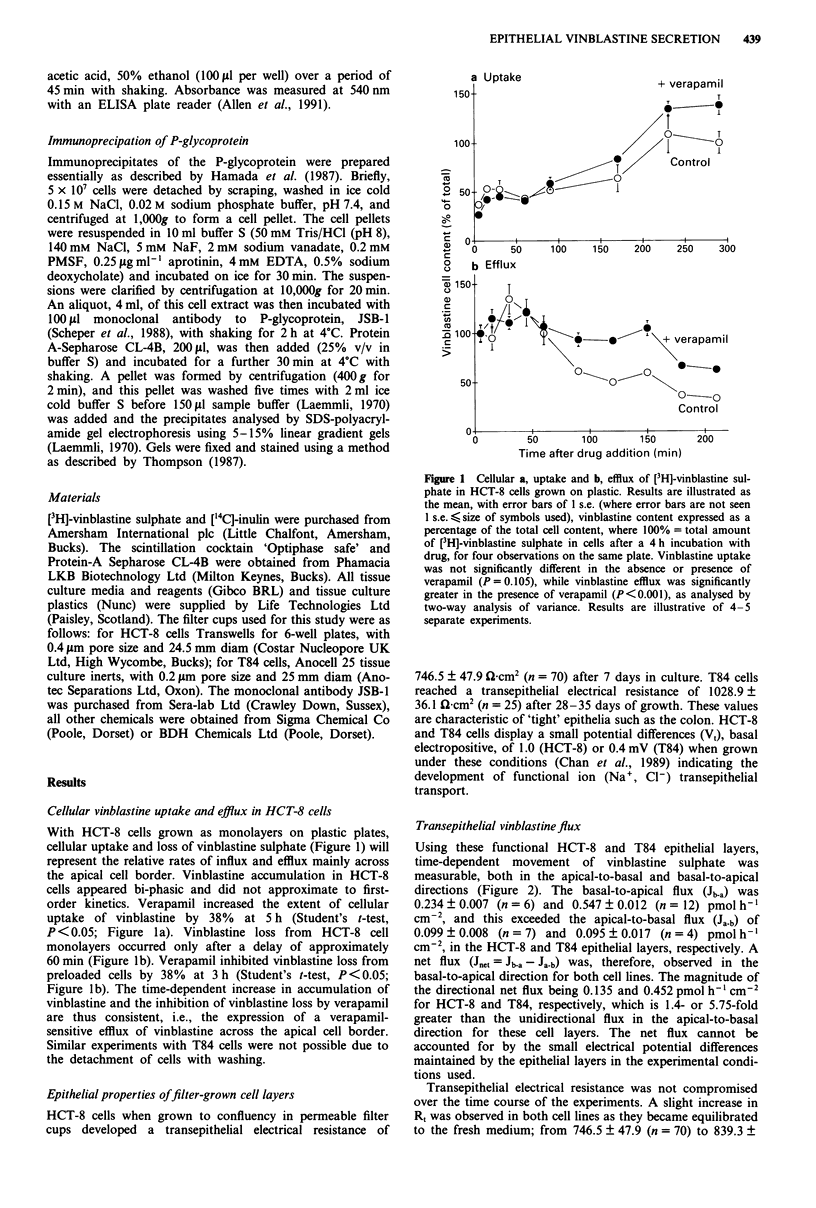
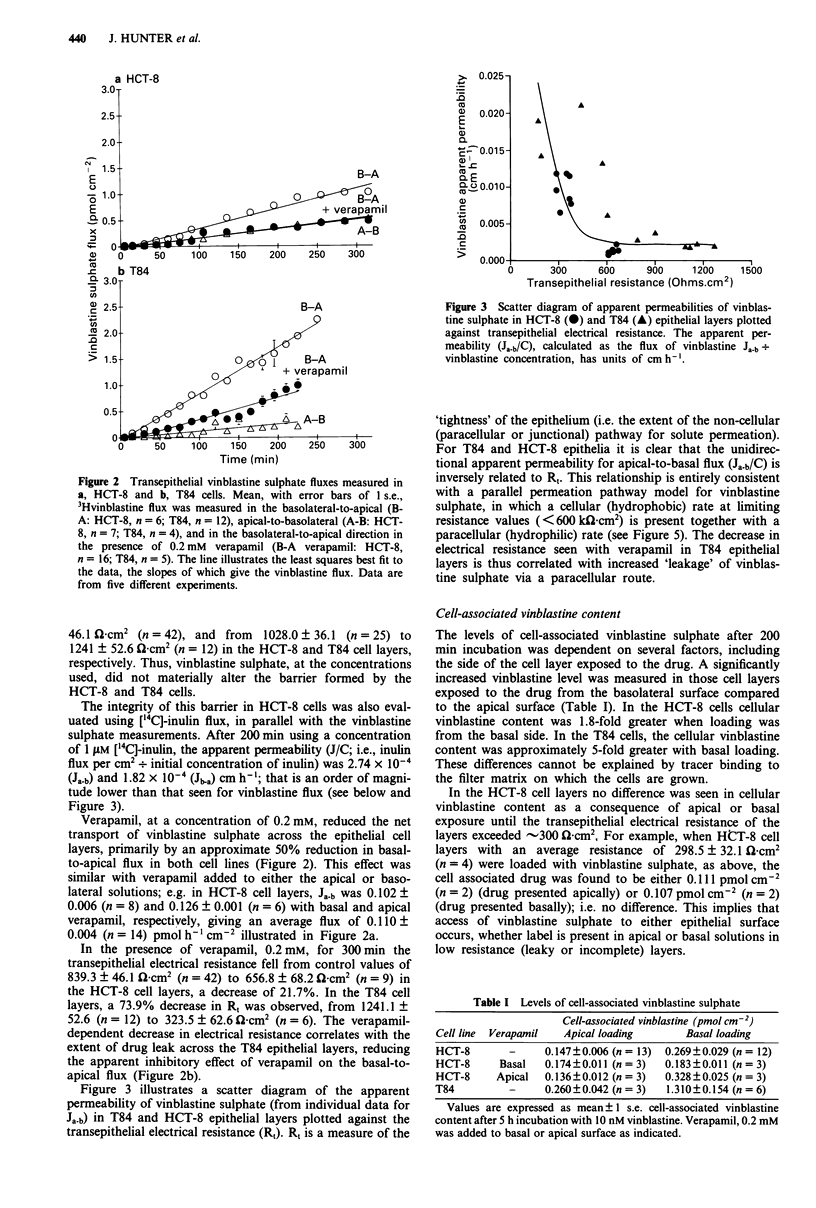
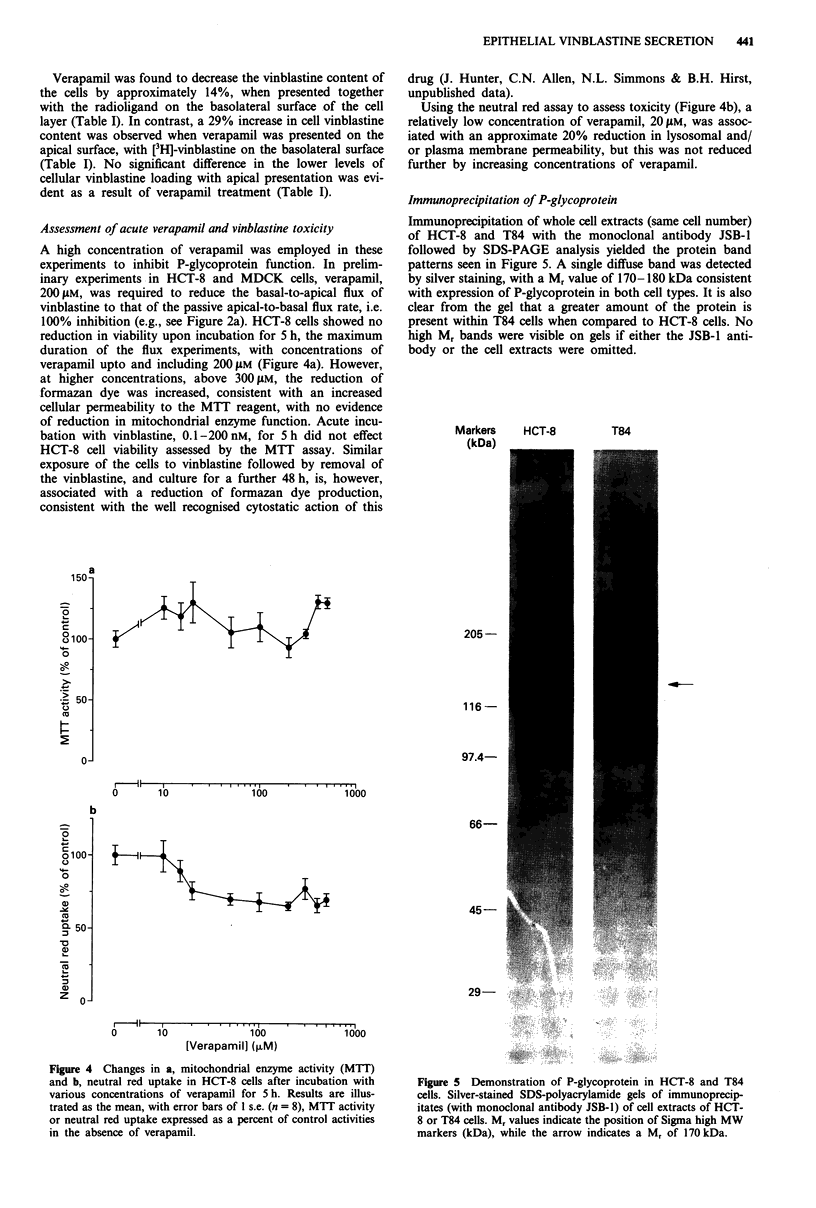
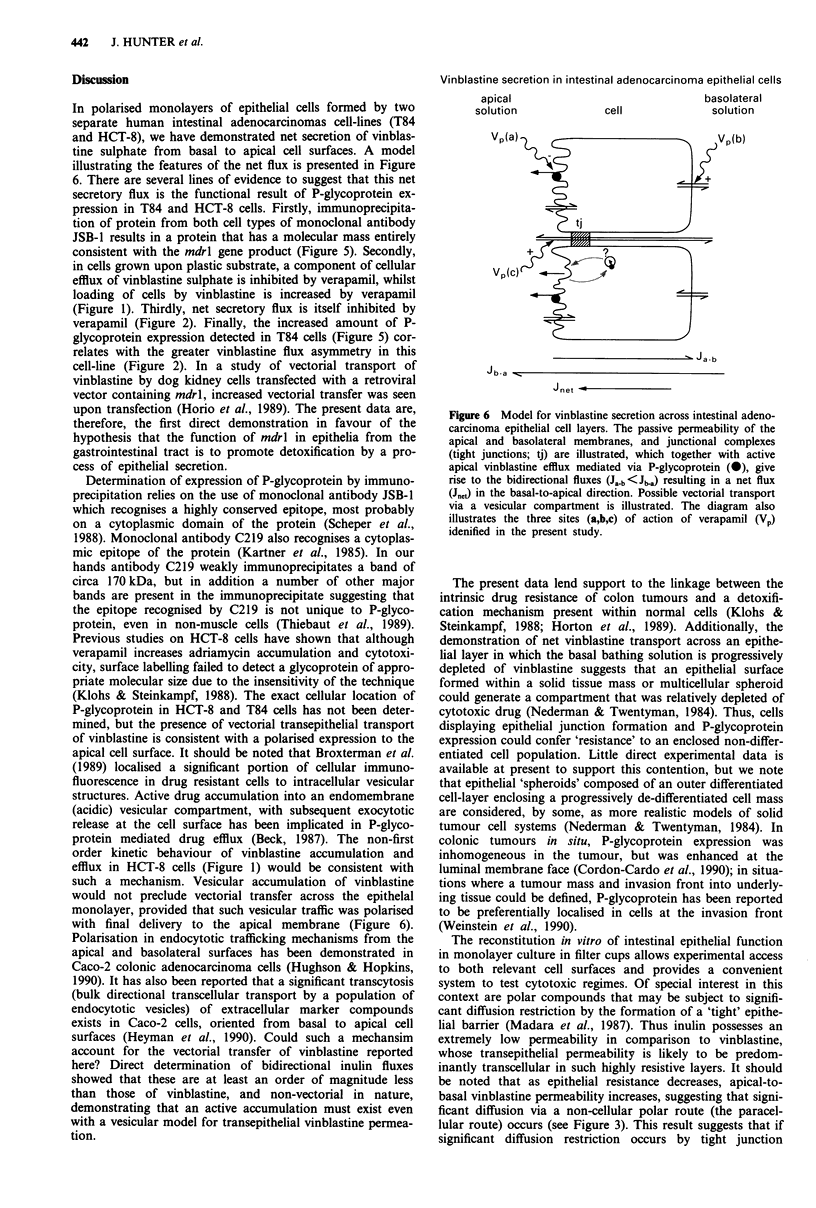
P-glycoprotein expression was demonstrated in two human intestinal adenocarcinoma cell-lines (HCT-8, ileocaecal and T84, colonic) by immunoprecipitation of a 170-180 kDa protein with monoclonal antibody JSB-1. Both HCT-8 and T84 formed functional epithelial cell layers of high transepithelial electrical resistance (greater than 700 omega.cm2) when grown on permeable matrices. These epithelial layers demonstrated vectorial secretion (net vinblastine fluxes in the basal-to-apical direction of 0.135 and 0.452 pmol h-1 cm-2 in HCT-8 and T84 cell layers, respectively, from bathing solutions containing 10 nM vinblastine). These vectorial vinblastine secretions were sensitive to inhibition by verapamil. Passive transepithelial vinblastine permeation was limited by the presence of intercellular (tight) junctions, as demonstrated by the high transepithelial electrical resistance, and verapamil increased this passive vinblastine permeation concomitant with a reduction in the electrical resistance. Cellular vinblastine loading was significantly greater from the basal side, and this was also susceptible to inhibition by basal verapamil. The demonstration of vectorial transport of vinblastine in human intestinal colonic adenocarcinoma cell layers is direct evidence in favour of the hypothesis that the function of mdr1 in epithelial from the gastrointestinal tract is to promote detoxification by a process of epithelial secretion. This study also highlights that cellular vinblastine accumulation depends not only upon P-glycoprotein function, but also upon differential apparent membrane permeabilities and the presence of intercellular (tight) junctions that may restrict drug permeation and cellular accumulation to apical or basal membrane domains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C. N., Harpur E. S., Gray T. J., Simmons N. L., Hirst B. H. Efflux of bis-carboxyethyl-carboxyfluorescein (BCECF) by a novel ATP-dependent transport mechanism in epithelial cells. Biochem Biophys Res Commun. 1990 Oct 15;172(1):262–267. doi: 10.1016/s0006-291x(05)80203-7. [DOI] [PubMed] [Google Scholar]
- Beck W. T. The cell biology of multiple drug resistance. Biochem Pharmacol. 1987 Sep 15;36(18):2879–2887. doi: 10.1016/0006-2952(87)90198-5. [DOI] [PubMed] [Google Scholar]
- Broxterman H. J., Pinedo H. M., Kuiper C. M., van der Hoeven J. J., de Lange P., Quak J. J., Scheper R. J., Keizer H. G., Schuurhuis G. J., Lankelma J. Immunohistochemical detection of P-glycoprotein in human tumor cells with a low degree of drug resistance. Int J Cancer. 1989 Feb 15;43(2):340–343. doi: 10.1002/ijc.2910430229. [DOI] [PubMed] [Google Scholar]
- Chan A. B., Allen C. N., Simmons N. L., Parsons M. E., Hirst B. H. Resistance to acid of canine kidney (MDCK) and human colonic (T84) and ileo-caecal (HCT-8) adenocarcinoma epithelial cell monolayers in vitro. Q J Exp Physiol. 1989 Jul;74(4):553–556. doi: 10.1113/expphysiol.1989.sp003304. [DOI] [PubMed] [Google Scholar]
- Chantret I., Barbat A., Dussaulx E., Brattain M. G., Zweibaum A. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res. 1988 Apr 1;48(7):1936–1942. [PubMed] [Google Scholar]
- Cordon-Cardo C., O'Brien J. P., Boccia J., Casals D., Bertino J. R., Melamed M. R. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem. 1990 Sep;38(9):1277–1287. doi: 10.1177/38.9.1974900. [DOI] [PubMed] [Google Scholar]
- Cornwell M. M., Pastan I., Gottesman M. M. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J Biol Chem. 1987 Feb 15;262(5):2166–2170. [PubMed] [Google Scholar]
- Cornwell M. M., Safa A. R., Felsted R. L., Gottesman M. M., Pastan I. Membrane vesicles from multidrug-resistant human cancer cells contain a specific 150- to 170-kDa protein detected by photoaffinity labeling. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3847–3850. doi: 10.1073/pnas.83.11.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973 Oct 25;323(3):466–483. doi: 10.1016/0005-2736(73)90191-0. [DOI] [PubMed] [Google Scholar]
- Deffie A. M., Alam T., Seneviratne C., Beenken S. W., Batra J. K., Shea T. C., Henner W. D., Goldenberg G. J. Multifactorial resistance to adriamycin: relationship of DNA repair, glutathione transferase activity, drug efflux, and P-glycoprotein in cloned cell lines of adriamycin-sensitive and -resistant P388 leukemia. Cancer Res. 1988 Jul 1;48(13):3595–3602. [PubMed] [Google Scholar]
- Dharmsathaphorn K., McRoberts J. A., Mandel K. G., Tisdale L. D., Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984 Feb;246(2 Pt 1):G204–G208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- Fogler W. E., Klinger M. R., Abraham K. G., Gottlinger H. G., Riethmuller G., Daddona P. E. Enhanced cytotoxicity against colon carcinoma by combinations of noncompeting monoclonal antibodies to the 17-1A antigen. Cancer Res. 1988 Nov 15;48(22):6303–6308. [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J. M., Hait W. N. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev. 1990 Sep;42(3):155–199. [PubMed] [Google Scholar]
- Goldstein L. J., Galski H., Fojo A., Willingham M., Lai S. L., Gazdar A., Pirker R., Green A., Crist W., Brodeur G. M. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst. 1989 Jan 18;81(2):116–124. doi: 10.1093/jnci/81.2.116. [DOI] [PubMed] [Google Scholar]
- Hamada H., Hagiwara K., Nakajima T., Tsuruo T. Phosphorylation of the Mr 170,000 to 180,000 glycoprotein specific to multidrug-resistant tumor cells: effects of verapamil, trifluoperazine, and phorbol esters. Cancer Res. 1987 Jun 1;47(11):2860–2865. [PubMed] [Google Scholar]
- Heyman M., Crain-Denoyelle A. M., Nath S. K., Desjeux J. F. Quantification of protein transcytosis in the human colon carcinoma cell line CaCo-2. J Cell Physiol. 1990 May;143(2):391–395. doi: 10.1002/jcp.1041430225. [DOI] [PubMed] [Google Scholar]
- Horio M., Chin K. V., Currier S. J., Goldenberg S., Williams C., Pastan I., Gottesman M. M., Handler J. Transepithelial transport of drugs by the multidrug transporter in cultured Madin-Darby canine kidney cell epithelia. J Biol Chem. 1989 Sep 5;264(25):14880–14884. [PubMed] [Google Scholar]
- Horio M., Lovelace E., Pastan I., Gottesman M. M. Agents which reverse multidrug-resistance are inhibitors of [3H]vinblastine transport by isolated vesicles. Biochim Biophys Acta. 1991 Jan 9;1061(1):106–110. doi: 10.1016/0005-2736(91)90274-c. [DOI] [PubMed] [Google Scholar]
- Horton J. K., Thimmaiah K. N., Houghton J. A., Horowitz M. E., Houghton P. J. Modulation by verapamil of vincristine pharmacokinetics and toxicity in mice bearing human tumor xenografts. Biochem Pharmacol. 1989 Jun 1;38(11):1727–1736. doi: 10.1016/0006-2952(89)90405-x. [DOI] [PubMed] [Google Scholar]
- Hughson E. J., Hopkins C. R. Endocytic pathways in polarized Caco-2 cells: identification of an endosomal compartment accessible from both apical and basolateral surfaces. J Cell Biol. 1990 Feb;110(2):337–348. doi: 10.1083/jcb.110.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba M., Kobayashi H., Sakurai Y., Johnson R. K. Active efflux of daunorubicin and adriamycin in sensitive and resistant sublines of P388 leukemia. Cancer Res. 1979 Jun;39(6 Pt 1):2200–2203. [PubMed] [Google Scholar]
- Kartner N., Evernden-Porelle D., Bradley G., Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. 1985 Aug 29-Sep 4Nature. 316(6031):820–823. doi: 10.1038/316820a0. [DOI] [PubMed] [Google Scholar]
- Keizer H. G., Schuurhuis G. J., Broxterman H. J., Lankelma J., Schoonen W. G., van Rijn J., Pinedo H. M., Joenje H. Correlation of multidrug resistance with decreased drug accumulation, altered subcellular drug distribution, and increased P-glycoprotein expression in cultured SW-1573 human lung tumor cells. Cancer Res. 1989 Jun 1;49(11):2988–2993. [PubMed] [Google Scholar]
- Kessel D., Wilberding C. Mode of action of calcium antagonists which alter anthracycline resistance. Biochem Pharmacol. 1984 Apr 1;33(7):1157–1160. doi: 10.1016/0006-2952(84)90533-1. [DOI] [PubMed] [Google Scholar]
- Kessel D., Wilberding C. Promotion of daunorubicin uptake and toxicity by the calcium antagonist tiapamil and its analogs. Cancer Treat Rep. 1985 Jun;69(6):673–676. [PubMed] [Google Scholar]
- Klohs W. D., Steinkampf R. W. Possible link between the intrinsic drug resistance of colon tumors and a detoxification mechanism of intestinal cells. Cancer Res. 1988 Jun 1;48(11):3025–3030. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. F., Ogden P., Simmons N. L. Autoradiographic localisation of [3H]ouabain bound to cultured epithelial cell monolayers of MDCK cells. Biochim Biophys Acta. 1981 Jun 22;644(2):333–340. doi: 10.1016/0005-2736(81)90391-6. [DOI] [PubMed] [Google Scholar]
- Leukaemia Research Fund international research symposium on cytotoxic drug resistance in leukemia and other malignancies. Leukemia. 1989 Jun;3(6):461–467. [PubMed] [Google Scholar]
- Madara J. L., Dharmsathaphorn K. Occluding junction structure-function relationships in a cultured epithelial monolayer. J Cell Biol. 1985 Dec;101(6):2124–2133. doi: 10.1083/jcb.101.6.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederman T., Twentyman P. Spheroids for studies of drug effects. Recent Results Cancer Res. 1984;95:84–102. doi: 10.1007/978-3-642-82340-4_5. [DOI] [PubMed] [Google Scholar]
- Pastan I., Gottesman M. M., Ueda K., Lovelace E., Rutherford A. V., Willingham M. C. A retrovirus carrying an MDR1 cDNA confers multidrug resistance and polarized expression of P-glycoprotein in MDCK cells. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4486–4490. doi: 10.1073/pnas.85.12.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safa A. R., Glover C. J., Meyers M. B., Biedler J. L., Felsted R. L. Vinblastine photoaffinity labeling of a high molecular weight surface membrane glycoprotein specific for multidrug-resistant cells. J Biol Chem. 1986 May 15;261(14):6137–6140. [PubMed] [Google Scholar]
- Safa A. R., Glover C. J., Sewell J. L., Meyers M. B., Biedler J. L., Felsted R. L. Identification of the multidrug resistance-related membrane glycoprotein as an acceptor for calcium channel blockers. J Biol Chem. 1987 Jun 5;262(16):7884–7888. [PubMed] [Google Scholar]
- Scheper R. J., Bulte J. W., Brakkee J. G., Quak J. J., van der Schoot E., Balm A. J., Meijer C. J., Broxterman H. J., Kuiper C. M., Lankelma J. Monoclonal antibody JSB-1 detects a highly conserved epitope on the P-glycoprotein associated with multi-drug-resistance. Int J Cancer. 1988 Sep 15;42(3):389–394. doi: 10.1002/ijc.2910420314. [DOI] [PubMed] [Google Scholar]
- Simmons N. L. Tissue culture of established renal cell lines. Methods Enzymol. 1990;191:426–436. doi: 10.1016/0076-6879(90)91027-4. [DOI] [PubMed] [Google Scholar]
- Simons K., Fuller S. D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Sirotnak F. M., O'Leary D. F. The issues of transport multiplicity and energetics pertaining to methotrexate efflux in L1210 cells addressed by an analysis of cis and trans effects of inhibitors. Cancer Res. 1991 Mar 1;51(5):1412–1417. [PubMed] [Google Scholar]
- Skovsgaard T. Mechanism of cross-resistance between vincristine and daunorubicin in Ehrlich ascites tumor cells. Cancer Res. 1978 Dec;38(12):4722–4727. [PubMed] [Google Scholar]
- Skovsgaard T. Mechanisms of resistance to daunorubicin in Ehrlich ascites tumor cells. Cancer Res. 1978 Jun;38(6):1785–1791. [PubMed] [Google Scholar]
- Sugawara I., Kataoka I., Morishita Y., Hamada H., Tsuruo T., Itoyama S., Mori S. Tissue distribution of P-glycoprotein encoded by a multidrug-resistant gene as revealed by a monoclonal antibody, MRK 16. Cancer Res. 1988 Apr 1;48(7):1926–1929. [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Immunohistochemical localization in normal tissues of different epitopes in the multidrug transport protein P170: evidence for localization in brain capillaries and crossreactivity of one antibody with a muscle protein. J Histochem Cytochem. 1989 Feb;37(2):159–164. doi: 10.1177/37.2.2463300. [DOI] [PubMed] [Google Scholar]
- Tompkins W. A., Watrach A. M., Schmale J. D., Schultz R. M., Harris J. A. Cultural and antigenic properties of newly established cell strains derived from adenocarcinomas of the human colon and rectum. J Natl Cancer Inst. 1974 Apr;52(4):1101–1110. doi: 10.1093/jnci/52.4.1101. [DOI] [PubMed] [Google Scholar]
- Tsuruo T. Reversal of acquired resistance to vinca alkaloids and anthracycline antibiotics. Cancer Treat Rep. 1983 Oct;67(10):889–894. [PubMed] [Google Scholar]
- Twentyman P. R., Fox N. E., Bleehen N. M. Drug resistance in human lung cancer cell lines: cross-resistance studies and effects of the calcium transport blocker, verapamil. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1355–1358. doi: 10.1016/0360-3016(86)90170-7. [DOI] [PubMed] [Google Scholar]
- Ueda K., Cardarelli C., Gottesman M. M., Pastan I. Expression of a full-length cDNA for the human "MDR1" gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci U S A. 1987 May;84(9):3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Bliek A. M., Baas F., Van der Velde-Koerts T., Biedler J. L., Meyers M. B., Ozols R. F., Hamilton T. C., Joenje H., Borst P. Genes amplified and overexpressed in human multidrug-resistant cell lines. Cancer Res. 1988 Nov 1;48(21):5927–5932. [PubMed] [Google Scholar]
- Weinstein R. S., Kuszak J. R., Kluskens L. F., Coon J. S. P-glycoproteins in pathology: the multidrug resistance gene family in humans. Hum Pathol. 1990 Jan;21(1):34–48. doi: 10.1016/0046-8177(90)90073-e. [DOI] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Fuller S. D., Simons K. Apical and basolateral endocytosis in Madin-Darby canine kidney (MDCK) cells grown on nitrocellulose filters. EMBO J. 1985 Nov;4(11):2781–2792. doi: 10.1002/j.1460-2075.1985.tb04004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]