Abstract
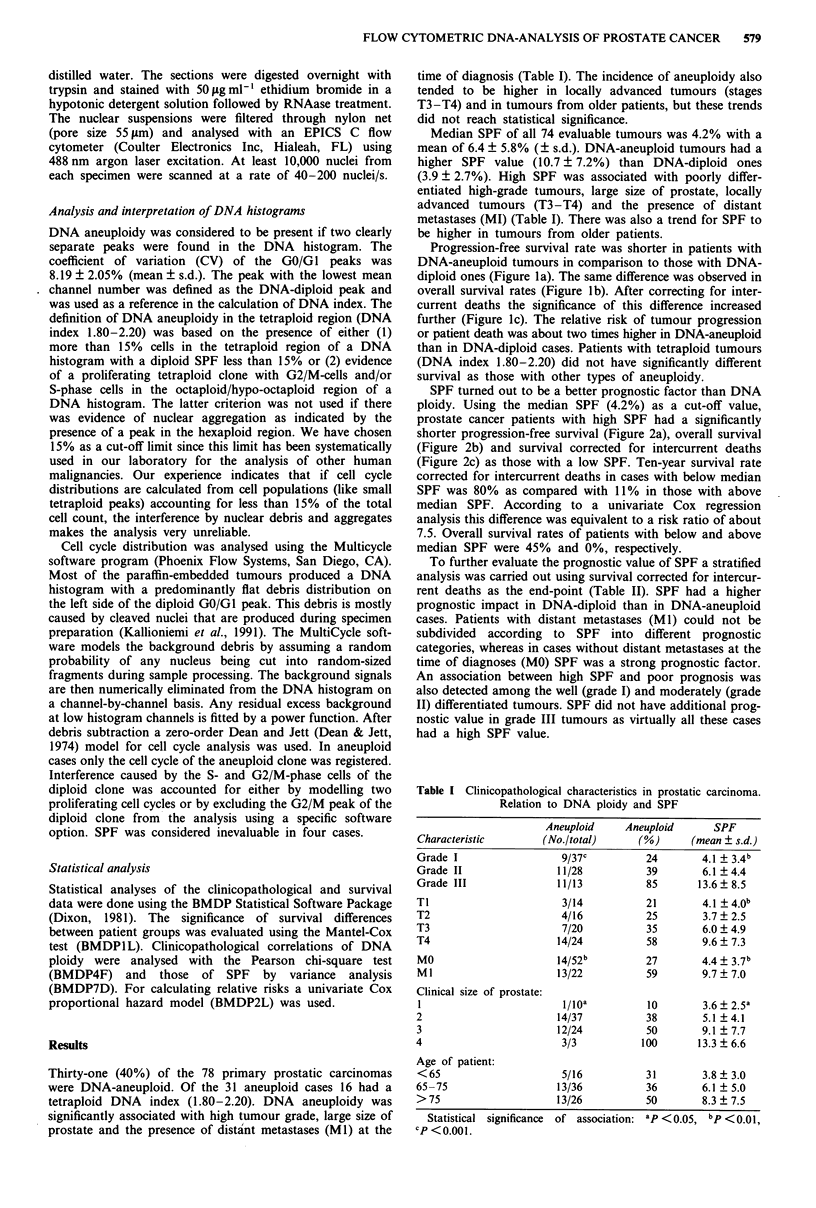
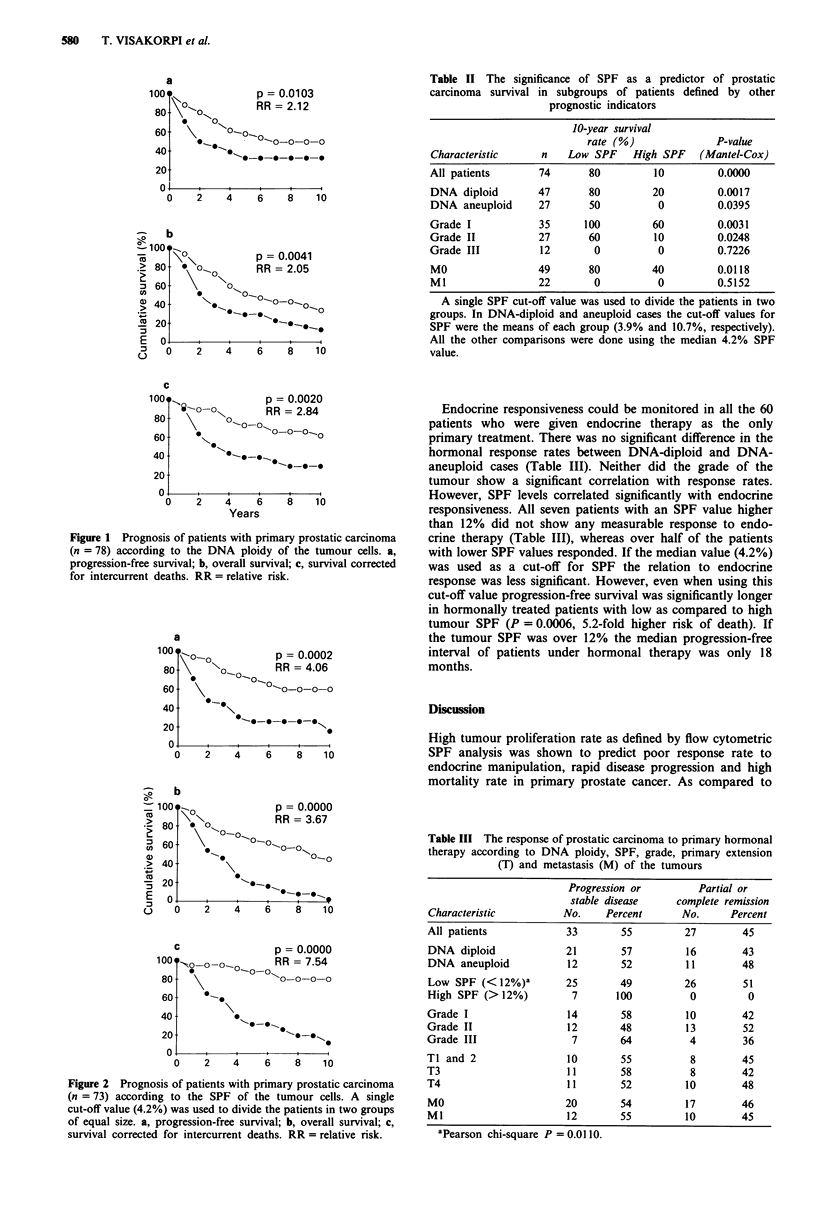
We analysed ploidy and S-phase fraction (SPF) from 78 paraffin-embedded primary prostatic carcinomas by DNA flow cytometry. DNA aneuploidy and above median (4.2%) SPF were both associated with high tumour grade, large size of prostate and presence of distant metastases. Both aneuploidy and high SPF (greater than 4.2%) indicated short 10-year progression-free (P = 0.01 for ploidy and P = 0.0002 for SPF), overall (P = 0.004 and P less than 0.0001) as well as prostate cancer survival (P = 0.002 and P less than 0.0001). Ten-year overall survival rate was 45% in cases with SPF below 4.2% and 0% in those with higher values, whereas the corresponding prostate cancer-specific survival rates were 80% and 11%, respectively. None of the seven tumours with SPF above 12% showed an objective response to endocrine therapy, whereas 26/49 (52%) of those with lower SPF values responded (P = 0.01). DNA ploidy, tumour grade, T-stage or M-stage did not significantly correlate with endocrine responsiveness. SPF was also the best predictor of progression free survival in patients treated hormonally. In conclusion, detection of high SPF in prostate cancer may indicate lack of hormonal responsiveness and poor prognosis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolfsson J., Rönström L., Hedlund P. O., Löwhagen T., Carstensen J., Tribukait B. The prognostic value of modal deoxyribonucleic acid in low grade, low stage untreated prostate cancer. J Urol. 1990 Dec;144(6):1404–1407. doi: 10.1016/s0022-5347(17)39754-9. [DOI] [PubMed] [Google Scholar]
- Dawson A. E., Norton J. A., Weinberg D. S. Comparative assessment of proliferation and DNA content in breast carcinoma by image analysis and flow cytometry. Am J Pathol. 1990 May;136(5):1115–1124. [PMC free article] [PubMed] [Google Scholar]
- Dean P. N., Jett J. H. Mathematical analysis of DNA distributions derived from flow microfluorometry. J Cell Biol. 1974 Feb;60(2):523–527. doi: 10.1083/jcb.60.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejter S. W., Jr, Cunningham R. E., Noguchi P. D., Jones R. V., Moul J. W., McLeod D. G., Lynch J. H. Prognostic significance of DNA ploidy in carcinoma of prostate. Urology. 1989 May;33(5):361–366. doi: 10.1016/0090-4295(89)90026-5. [DOI] [PubMed] [Google Scholar]
- Frankfurt O. S., Greco W. R., Slocum H. K., Arbuck S. G., Gamarra M., Pavelic Z. P., Rustum Y. M. Proliferative characteristics of primary and metastatic human solid tumors by DNA flow cytometry. Cytometry. 1984 Nov;5(6):629–635. doi: 10.1002/cyto.990050612. [DOI] [PubMed] [Google Scholar]
- Haugen O. A., Mjølnerød O. DNA-ploidy as prognostic factor in prostatic carcinoma. Int J Cancer. 1990 Feb 15;45(2):224–228. doi: 10.1002/ijc.2910450203. [DOI] [PubMed] [Google Scholar]
- Hedley D. W. Flow cytometry using paraffin-embedded tissue: five years on. Cytometry. 1989 May;10(3):229–241. doi: 10.1002/cyto.990100302. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W., Rugg C. A., Musgrove E. A. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem. 1983 Nov;31(11):1333–1335. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Rugg C. A., Gelber R. D. Association of DNA index and S-phase fraction with prognosis of nodes positive early breast cancer. Cancer Res. 1987 Sep 1;47(17):4729–4735. [PubMed] [Google Scholar]
- Horoszewicz J. S., Leong S. S., Kawinski E., Karr J. P., Rosenthal H., Chu T. M., Mirand E. A., Murphy G. P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983 Apr;43(4):1809–1818. [PubMed] [Google Scholar]
- Kallioniemi O. P. Comparison of fresh and paraffin-embedded tissue as starting material for DNA flow cytometry and evaluation of intratumor heterogeneity. Cytometry. 1988 Mar;9(2):164–169. doi: 10.1002/cyto.990090211. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O. P., Visakorpi T., Holli K., Heikkinen A., Isola J., Koivula T. Improved prognostic impact of S-phase values from paraffin-embedded breast and prostate carcinomas after correcting for nuclear slicing. Cytometry. 1991;12(5):413–421. doi: 10.1002/cyto.990120506. [DOI] [PubMed] [Google Scholar]
- Lee S. E., Currin S. M., Paulson D. F., Walther P. J. Flow cytometric determination of ploidy in prostatic adenocarcinoma: a comparison with seminal vesicle involvement and histopathological grading as a predictor of clinical recurrence. J Urol. 1988 Oct;140(4):769–774. doi: 10.1016/s0022-5347(17)41808-8. [DOI] [PubMed] [Google Scholar]
- McDivitt R. W., Stone K. R., Craig R. B., Palmer J. O., Meyer J. S., Bauer W. C. A proposed classification of breast cancer based on kinetic information: derived from a comparison of risk factors in 168 primary operable breast cancers. Cancer. 1986 Jan 15;57(2):269–276. doi: 10.1002/1097-0142(19860115)57:2<269::aid-cncr2820570214>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Merkel D. E., McGuire W. L. Ploidy, proliferative activity and prognosis. DNA flow cytometry of solid tumors. Cancer. 1990 Mar 1;65(5):1194–1205. doi: 10.1002/1097-0142(19900301)65:5<1194::aid-cncr2820650528>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Montgomery B. T., Nativ O., Blute M. L., Farrow G. M., Myers R. P., Zincke H., Therneau T. M., Lieber M. M. Stage B prostate adenocarcinoma. Flow cytometric nuclear DNA ploidy analysis. Arch Surg. 1990 Mar;125(3):327–331. doi: 10.1001/archsurg.1990.01410150049010. [DOI] [PubMed] [Google Scholar]
- Morris G. L., Dodd J. G. Epidermal growth factor receptor mRNA levels in human prostatic tumors and cell lines. J Urol. 1990 Jun;143(6):1272–1274. doi: 10.1016/s0022-5347(17)40253-9. [DOI] [PubMed] [Google Scholar]
- Nativ O., Winkler H. Z., Raz Y., Therneau T. M., Farrow G. M., Myers R. P., Zincke H., Lieber M. M. Stage C prostatic adenocarcinoma: flow cytometric nuclear DNA ploidy analysis. Mayo Clin Proc. 1989 Aug;64(8):911–919. doi: 10.1016/s0025-6196(12)61218-x. [DOI] [PubMed] [Google Scholar]
- Neill W. A., Norval M., Habib F. K. Nuclear DNA analysis of prostate tissues: correlation with stage and grade of tumour. Urol Int. 1989;44(3):141–146. doi: 10.1159/000281490. [DOI] [PubMed] [Google Scholar]
- Schultz R. E., Varello M. A., Tsou K. C., Wein A. J., Murphy J. J. Simultaneous flow cytometric deoxyribonucleic acid and acid phosphatase analysis of benign and malignant lesions of the prostate. J Urol. 1985 Dec;134(6):1133–1136. doi: 10.1016/s0022-5347(17)47658-0. [DOI] [PubMed] [Google Scholar]
- Schulze H., Isaacs J. T. Biology and therapy of prostatic cancer. Cancer Surv. 1986;5(3):487–503. [PubMed] [Google Scholar]
- Stephenson R. A., James B. C., Gay H., Fair W. R., Whitmore W. F., Jr, Melamed M. R. Flow cytometry of prostate cancer: relationship of DNA content to survival. Cancer Res. 1987 May 1;47(9):2504–2507. [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J. A review of techniques and results obtained in one laboratory by an integrated system of methods designed for routine clinical flow cytometric DNA analysis. Cytometry. 1990;11(7):753–770. doi: 10.1002/cyto.990110702. [DOI] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J., Nissen N. I. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983 Mar;3(5):323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- Winkler H. Z., Rainwater L. M., Myers R. P., Farrow G. M., Therneau T. M., Zincke H., Lieber M. M. Stage D1 prostatic adenocarcinoma: significance of nuclear DNA ploidy patterns studied by flow cytometry. Mayo Clin Proc. 1988 Feb;63(2):103–112. doi: 10.1016/s0025-6196(12)64942-8. [DOI] [PubMed] [Google Scholar]


