Abstract
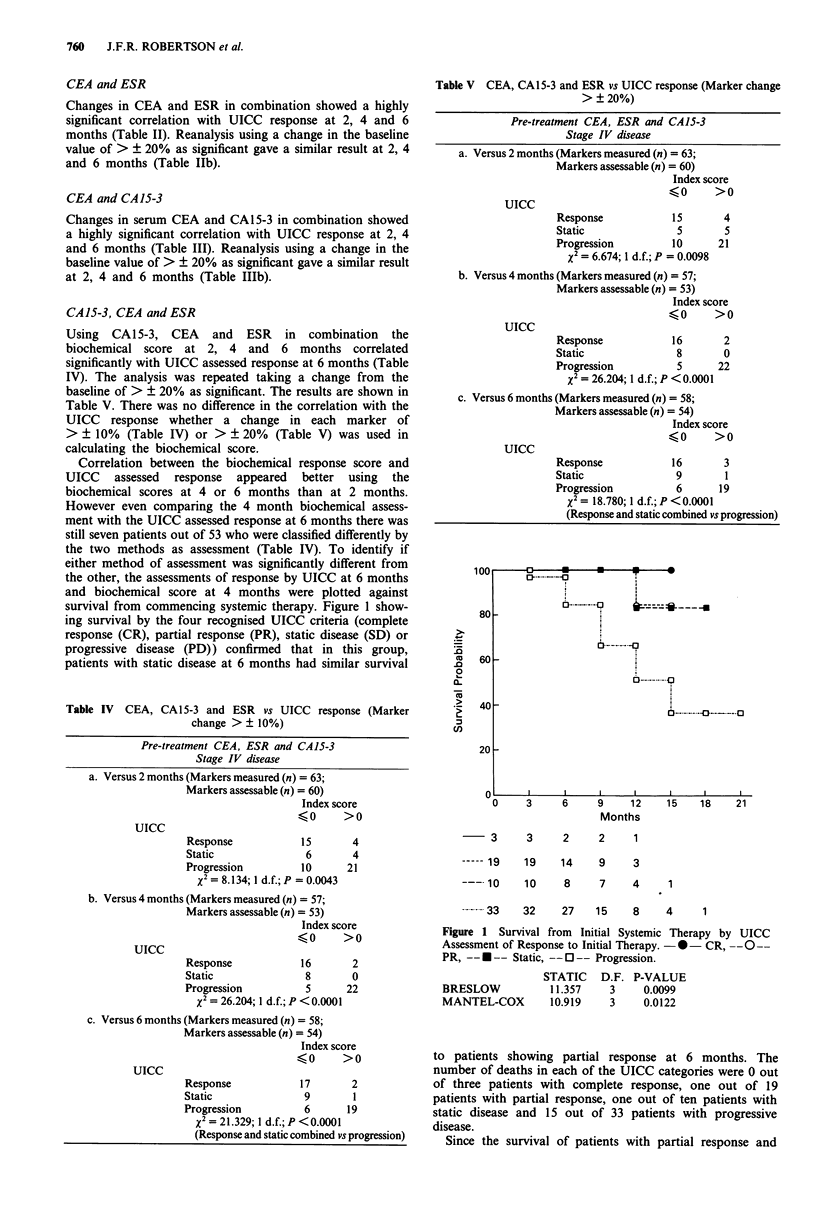
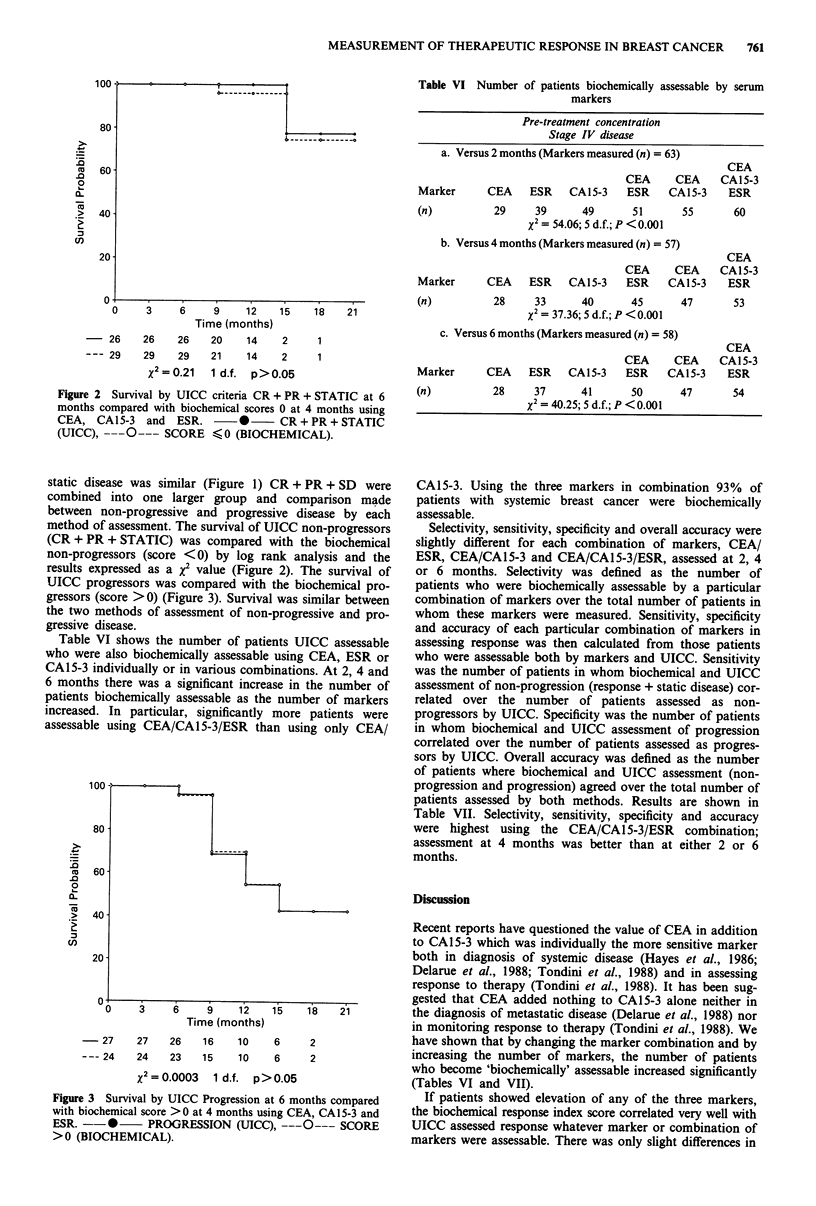
In 65 patients with systemic breast cancer, a biochemical response index using three tumour markers in combination, carcinoembryonic antigen (CEA), carbohydrate antigen 15-3 (CA 15-3) and erythrocyte sedimentation rate (ESR), allowed objective biochemical assessment of response to endocrine therapy. Changes in these three markers at 2, 4 and 6 months showed a highly significant correlation with UICC assessed response at 6 months. At 4 months, changes in these three markers resulted in a selectivity of 93%, with a sensitivity of 92% and a specificity of 82%. Survival of groups of patients assessed biochemically or by UICC criteria for non-progression or progression showed no significant difference. The advantage of the biochemical assessment are that it is objective and reproducible. The assessment gives similar information to the UICC assessment but can be carried out earlier. Changes in the three markers appears to reflect the dynamics of change in tumour mass in response to systemic therapy in contrast to the UICC criteria which reflect structural change.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bezwoda W., Derman D., Bothwell T., MacPhil P., Levin J., De Moor N. Significance of serum concentrations of carcinoembryonic antigen, ferritin, and calcitonin in breast cancer. Cancer. 1981 Oct 1;48(7):1623–1628. doi: 10.1002/1097-0142(19811001)48:7<1623::aid-cncr2820480725>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Bray K. R., Koda J. E., Gaur P. K. Serum levels and biochemical characteristics of cancer-associated antigen CA-549, a circulating breast cancer marker. Cancer Res. 1987 Nov 15;47(22):5853–5860. [PubMed] [Google Scholar]
- Coombes R. C., Powles T. J., Gazet J. C., Ford H. T., McKinna A., Abbott M., Gehrke C. W., Keyser J. W., Mitchell P. E., Patel S. Screening for metastases in breast cancer: an assessment of biochemical and physical methods. Cancer. 1981 Jul 15;48(2):310–315. doi: 10.1002/1097-0142(19810715)48:2<310::aid-cncr2820480216>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Coombes R. C., Powles T. J., Gazet J. C., Ford H. T., Nash A. G., Sloane J. P., Hillyard C. J., Thomas P., Keyser J. W., Marcus D. A biochemical approach to the staging of human breast cancer. Cancer. 1977 Aug;40(2):937–944. doi: 10.1002/1097-0142(197708)40:2<937::aid-cncr2820400252>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Cove D. H., Woods K. L., Smith S. C., Burnett D., Leonard J., Grieve R. J., Howell A. Tumour markers in breast cancer. Br J Cancer. 1979 Nov;40(5):710–718. doi: 10.1038/bjc.1979.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen D. M., Searle F., Ward A. M., Benson E. A., Smiddy F. G., Eaves G., Cooper E. H. Multivariate biochemical indicators of breast cancer: an evaluation of their potential in routine practice. Eur J Cancer. 1978 Aug;14(8):885–893. doi: 10.1016/0014-2964(78)90105-6. [DOI] [PubMed] [Google Scholar]
- Delarue J. C., Mouriesse H., Dubois F., Friedman S., May-Levin F. Markers in breast cancer: does CEA add to the detection by CA 15.3? Breast Cancer Res Treat. 1988 Jul;11(3):273–276. doi: 10.1007/BF01807287. [DOI] [PubMed] [Google Scholar]
- Franchimont P., Zangerle P. F., Nogarede J., Bury J., Molter F., Reuter A., Hendrick J. C., Collette J. Simultaneous assays of cancer-associated antigens in various neoplastic disorders. Cancer. 1976 Dec;38(6):2287–2295. doi: 10.1002/1097-0142(197612)38:6<2287::aid-cncr2820380616>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Fujino N., Haga Y., Sakamoto K., Egami H., Kimura M., Nishimura R., Akagi M. Clinical evaluation of an immunoradiometric assay for CA15-3 antigen associated with human mammary carcinomas: comparison with carcinoembryonic antigen. Jpn J Clin Oncol. 1986 Dec;16(4):335–346. [PubMed] [Google Scholar]
- Haagensen D. E., Jr, Kister S. J., Panick J., Giannola J., Hansen H. J., Wells S. A., Jr Comparative evaluation of carcinoembryonic antigen and gross cystic disease fluid protein as plasma markers for human breast carcinoma. Cancer. 1978 Sep;42(3 Suppl):1646–1652. doi: 10.1002/1097-0142(197809)42:3+<1646::aid-cncr2820420844>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Hayes D. F., Zurawski V. R., Jr, Kufe D. W. Comparison of circulating CA15-3 and carcinoembryonic antigen levels in patients with breast cancer. J Clin Oncol. 1986 Oct;4(10):1542–1550. doi: 10.1200/JCO.1986.4.10.1542. [DOI] [PubMed] [Google Scholar]
- Hayward J. L., Carbone P. P., Heuson J. C., Kumaoka S., Segaloff A., Rubens R. D. Assessment of response to therapy in advanced breast cancer: a project of the Programme on Clinical Oncology of the International Union Against Cancer, Geneva, Switzerland. Cancer. 1977 Mar;39(3):1289–1294. doi: 10.1002/1097-0142(197703)39:3<1289::aid-cncr2820390340>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Hilkens J., Bonfrer J. M., Kroezen V., van Eykeren M., Nooyen W., de Jong-Bakker M., Bruning P. F. Comparison of circulating MAM-6 and CEA levels and correlation with the estrogen receptor in patients with breast cancer. Int J Cancer. 1987 Apr 15;39(4):431–435. doi: 10.1002/ijc.2910390403. [DOI] [PubMed] [Google Scholar]
- Howell A., Mackintosh J., Jones M., Redford J., Wagstaff J., Sellwood R. A. The definition of the 'no change' category in patients treated with endocrine therapy and chemotherapy for advanced carcinoma of the breast. Eur J Cancer Clin Oncol. 1988 Oct;24(10):1567–1572. doi: 10.1016/0277-5379(88)90046-6. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O. P., Oksa H., Aaran R. K., Hietanen T., Lehtinen M., Koivula T. Serum CA 15-3 assay in the diagnosis and follow-up of breast cancer. Br J Cancer. 1988 Aug;58(2):213–215. doi: 10.1038/bjc.1988.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson R. I., Campbell F. C., Blamey R. W., Elston C. W., George D., Griffiths K. Steroid receptors in early breast cancer: value in prognosis. J Steroid Biochem. 1981 Dec;15:193–199. doi: 10.1016/0022-4731(81)90275-2. [DOI] [PubMed] [Google Scholar]
- Nicholson R. I., Colin P., Francis A. B., Keshra R., Finlay P., Williams M., Elston C. W., Blamey R. W., Griffiths K. Evaluation of an enzyme immunoassay for estrogen receptors in human breast cancers. Cancer Res. 1986 Aug;46(8 Suppl):4299s–4302s. [PubMed] [Google Scholar]
- Pons-Anicet D. M., Krebs B. P., Mira R., Namer M. Value of CA 15:3 in the follow-up of breast cancer patients. Br J Cancer. 1987 May;55(5):567–569. doi: 10.1038/bjc.1987.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. F., Pearson D., Price M. R., Selby C., Badley R. A., Pearson J., Blamey R. W., Howell A. Assessment of four monoclonal antibodies as serum markers in breast cancer. Eur J Cancer. 1990;26(11-12):1127–1132. doi: 10.1016/0277-5379(90)90268-x. [DOI] [PubMed] [Google Scholar]
- Robertson J. F., Williams M. R., Todd J., Nicholson R. I., Morgan D. A., Blamey R. W. Factors predicting the response of patients with advanced breast cancer to endocrine (Megace) therapy. Eur J Cancer Clin Oncol. 1989 Mar;25(3):469–475. doi: 10.1016/0277-5379(89)90259-9. [DOI] [PubMed] [Google Scholar]
- Silva J. S., Leight G. S., Haagensen D. E., Jr, Tallos P. B., Cox E. B., Dilley W. G., Wells S. A., Jr Quantitation of response to therapy in patients with metastatic breast carcinoma by serial analysis of plasma gross cystic disease fluid protein and carcinoembryonic antigen. Cancer. 1982 Mar 15;49(6):1236–1242. doi: 10.1002/1097-0142(19820315)49:6<1236::aid-cncr2820490627>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Stacker S. A., Sacks N. P., Golder J., Tjandra J. J., Thompson C. H., Smithyman A., McKenzie I. F. Evaluation of MSA as a serum marker in breast cancer: a comparison with CEA. Br J Cancer. 1988 Mar;57(3):298–303. doi: 10.1038/bjc.1988.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondini C., Hayes D. F., Gelman R., Henderson I. C., Kufe D. W. Comparison of CA15-3 and carcinoembryonic antigen in monitoring the clinical course of patients with metastatic breast cancer. Cancer Res. 1988 Jul 15;48(14):4107–4112. [PubMed] [Google Scholar]
- Waalkes T. P., Gehrke C. W., Tormey D. C., Woo K. B., Kuo K. C., Synder J., Hansen H. Biologic markers in breast carcinoma. IV. Serum fucose-protein ratio. Comparisons with carcinoembryonic antigen and human chorionic gonadotrophin. Cancer. 1978 May;41(5):1871–1882. doi: 10.1002/1097-0142(197805)41:5<1871::aid-cncr2820410531>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Williams M. R., Turkes A., Pearson D., Griffiths K., Blamey R. W. An objective biochemical assessment of therapeutic response in metastatic breast cancer: a study with external review of clinical data. Br J Cancer. 1990 Jan;61(1):126–132. doi: 10.1038/bjc.1990.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. B., Waalkes T. P., Ahmann D. L., Tormey D. C., Gehrke C. W., Oliverio V. T. A quantitative approach to determining disease response during therapy using multiple biologic markers: application to carcinoma of the breast. Cancer. 1978 May;41(5):1685–1703. doi: 10.1002/1097-0142(197805)41:5<1685::aid-cncr2820410507>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]


