Abstract
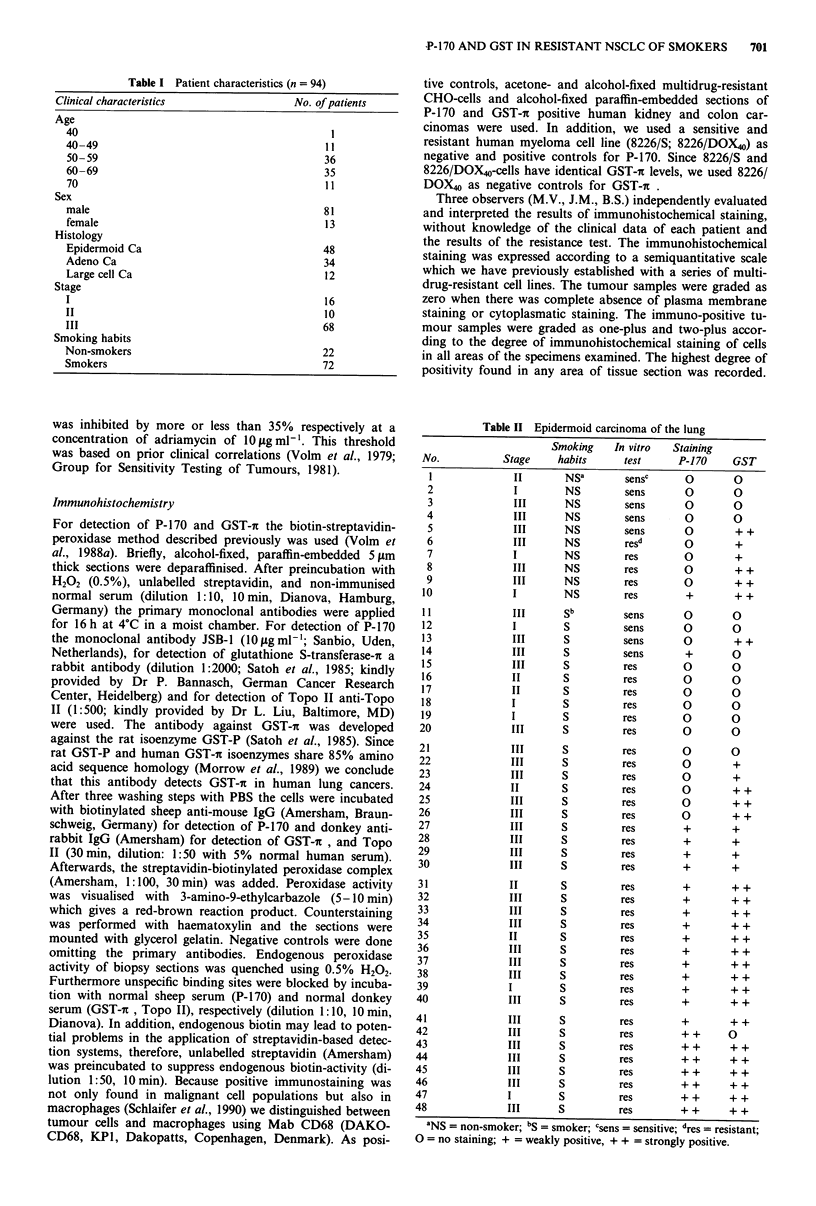
Ninety-four human non-small cell lung carcinomas (NSCLC) of previously untreated patients were analysed for the presence of P-glycoprotein (P-170) and glutathione S-transferase-pi (GST-pi) by means of immunohistochemistry. The expression of P-170 and GST-pi was compared with the results of doxorubicin resistance of the tumours in vitro and the smoking habits of the patients. A significant relationship between smoking habits of the patients and resistance of NSCLC was found (P = 0.007). Of the 72 tumours of smokers 57 (= 79%) were resistant, whereas of the 22 tumours of non-smokers only 11 (= 50%) showed resistance. Identical results were obtained when the analysis was restricted to patients with epidermoid lung carcinomas (P = 0.004). In contrast to these data, there exists no relationship between resistance and smoking for adenocarcinomas of the lung. Forty-two (= 58%) out of the 72 NSCLC of smokers expressed P-170, whereas out of 22 tumours of non-smokers only two tumours (= 9%) showed P-170 expression (P less than 0.0001). Similar results were obtained with epidermoid carcinomas (P = 0.004) and adenocarcinomas (P = 0.027). Fifty (= 69%) of 72 NSCLC of smokers revealed expression of GST-pi, whereas only nine (= 41%) of 22 tumours of non-smokers showed GST-pi expression (P = 0.015). Significant correlations also exist between resistance in vitro and expression of P-170 (P less than 0.0001) or expression of GST-pi (P less than 0.0001). Furthermore, a significant relationship between both proteins could be demonstrated (P less than 0.0001).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bak M., Jr, Efferth T., Mickisch G., Mattern J., Volm M. Detection of drug resistance and P-glycoprotein in human renal cell carcinomas. Eur Urol. 1990;17(1):72–75. doi: 10.1159/000464005. [DOI] [PubMed] [Google Scholar]
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Burt R. K., Garfield S., Johnson K., Thorgeirsson S. S. Transformation of rat liver epithelial cells with v-H-ras or v-raf causes expression of MDR-1, glutathione-S-transferase-P and increased resistance to cytotoxic chemicals. Carcinogenesis. 1988 Dec;9(12):2329–2332. doi: 10.1093/carcin/9.12.2329. [DOI] [PubMed] [Google Scholar]
- Carr B. I. Pleiotropic drug resistance in hepatocytes induced by carcinogens administered to rats. Cancer Res. 1987 Nov 1;47(21):5577–5583. [PubMed] [Google Scholar]
- Cole S. P., Downes H. F., Mirski S. E., Clements D. J. Alterations in glutathione and glutathione-related enzymes in a multidrug-resistant small cell lung cancer cell line. Mol Pharmacol. 1990 Feb;37(2):192–197. [PubMed] [Google Scholar]
- Cordon-Cardo C., O'Brien J. P., Boccia J., Casals D., Bertino J. R., Melamed M. R. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem. 1990 Sep;38(9):1277–1287. doi: 10.1177/38.9.1974900. [DOI] [PubMed] [Google Scholar]
- Cowan K. H., Batist G., Tulpule A., Sinha B. K., Myers C. E. Similar biochemical changes associated with multidrug resistance in human breast cancer cells and carcinogen-induced resistance to xenobiotics in rats. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9328–9332. doi: 10.1073/pnas.83.24.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffie A. M., Batra J. K., Goldenberg G. J. Direct correlation between DNA topoisomerase II activity and cytotoxicity in adriamycin-sensitive and -resistant P388 leukemia cell lines. Cancer Res. 1989 Jan 1;49(1):58–62. [PubMed] [Google Scholar]
- Fairchild C. R., Ivy S. P., Rushmore T., Lee G., Koo P., Goldsmith M. E., Myers C. E., Farber E., Cowan K. H. Carcinogen-induced mdr overexpression is associated with xenobiotic resistance in rat preneoplastic liver nodules and hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7701–7705. doi: 10.1073/pnas.84.21.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. The multidrug transporter, a double-edged sword. J Biol Chem. 1988 Sep 5;263(25):12163–12166. [PubMed] [Google Scholar]
- Gros P., Fallows D. A., Croop J. M., Housman D. E. Chromosome-mediated gene transfer of multidrug resistance. Mol Cell Biol. 1986 Nov;6(11):3785–3790. doi: 10.1128/mcb.6.11.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes J., Wareing C., Jacobs A., Hayes J. D., Padua R. A., Wolf C. R. Glutathione-s-transferase pi expression in leukaemia: a comparative analysis with mdr-1 data. Br J Cancer. 1990 Aug;62(2):209–212. doi: 10.1038/bjc.1990.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith W. N., Stallard S., Brown R. Expression of mdr1 and gst-pi in human breast tumours: comparison to in vitro chemosensitivity. Br J Cancer. 1990 May;61(5):712–716. doi: 10.1038/bjc.1990.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S. L., Goldstein L. J., Gottesman M. M., Pastan I., Tsai C. M., Johnson B. E., Mulshine J. L., Ihde D. C., Kayser K., Gazdar A. F. MDR1 gene expression in lung cancer. J Natl Cancer Inst. 1989 Aug 2;81(15):1144–1150. doi: 10.1093/jnci/81.15.1144. [DOI] [PubMed] [Google Scholar]
- Morrow C. S., Cowan K. H., Goldsmith M. E. Structure of the human genomic glutathione S-transferase-pi gene. Gene. 1989 Jan 30;75(1):3–11. doi: 10.1016/0378-1119(89)90377-6. [DOI] [PubMed] [Google Scholar]
- Radosevich J. A., Robinson P. G., Rittmann-Grauer L. S., Wilson B., Leung J. P., Maminta M. L., Warren W., Rosen S. T., Gould V. E. Immunohistochemical analysis of pulmonary and pleural tumors with the monoclonal antibody HYB-612 directed against the multidrug resistance (MDR-1) gene product, P-glycoprotein. Tumour Biol. 1989;10(5):252–257. doi: 10.1159/000217622. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Deuchars K., Kartner N., Alon N., Trent J., Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. 1985 Aug 29-Sep 4Nature. 316(6031):817–819. doi: 10.1038/316817a0. [DOI] [PubMed] [Google Scholar]
- Satoh K., Kitahara A., Soma Y., Inaba Y., Hatayama I., Sato K. Purification, induction, and distribution of placental glutathione transferase: a new marker enzyme for preneoplastic cells in the rat chemical hepatocarcinogenesis. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3964–3968. doi: 10.1073/pnas.82.12.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaifer D., Laurent G., Chittal S., Tsuruo T., Soues S., Muller C., Charcosset J. Y., Alard C., Brousset P., Mazerrolles C. Immunohistochemical detection of multidrug resistance associated P-glycoprotein in tumour and stromal cells of human cancers. Br J Cancer. 1990 Aug;62(2):177–182. doi: 10.1038/bjc.1990.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Bak M., Efferth T., Kaufmann M., Mattern J., Volm M. P-glycoprotein expression in treated and untreated human breast cancer. Br J Cancer. 1989 Dec;60(6):815–818. doi: 10.1038/bjc.1989.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y., Tsuruo T. DNA-mediated transfer and cloning of a human multidrug-resistant gene of adriamycin-resistant myelogenous leukemia K562. Cancer Res. 1987 May 15;47(10):2620–2625. [PubMed] [Google Scholar]
- Thorgeirsson S. S., Huber B. E., Sorrell S., Fojo A., Pastan I., Gottesman M. M. Expression of the multidrug-resistant gene in hepatocarcinogenesis and regenerating rat liver. Science. 1987 May 29;236(4805):1120–1122. doi: 10.1126/science.3576227. [DOI] [PubMed] [Google Scholar]
- Volm M., Bak M., Jr, Efferth T., Lathan B., Mattern J. Immunocytochemical detection of a resistance-associated glycoprotein in tissue culture cells, ascites tumors and human tumor xenografts by Mab 265/F4. Anticancer Res. 1988 Jul-Aug;8(4):531–535. [PubMed] [Google Scholar]
- Volm M., Bak M., Mattern J. Intrinsic drug resistance in a human lung carcinoma xenograft is associated with overexpression of multidrug-resistance DNA-sequences and of plasma membrane glycoproteins. Arzneimittelforschung. 1988 Aug;38(8):1189–1193. [PubMed] [Google Scholar]
- Volm M., Efferth T., Mattern J. Acquired drug resistance in human lung carcinoma xenografts. Arzneimittelforschung. 1989 Aug;39(8):828–831. [PubMed] [Google Scholar]
- Volm M., Samsel B., Mattern J. Relationship between chemoresistance of lung tumours and cigarette smoking. Br J Cancer. 1990 Aug;62(2):255–256. doi: 10.1038/bjc.1990.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volm M., Wayss K., Kaufmann M., Mattern J. Pretherapeutic detection of tumour resistance and the results of tumour chemotherapy. Eur J Cancer. 1979 Jul;15(7):983–993. doi: 10.1016/0014-2964(79)90282-2. [DOI] [PubMed] [Google Scholar]
- Volm M., Zerban H., Mattern J., Efferth T. Overexpression of P-glycoprotein in rat hepatocellular carcinomas induced with N-nitrosomorpholine. Carcinogenesis. 1990 Jan;11(1):169–172. doi: 10.1093/carcin/11.1.169. [DOI] [PubMed] [Google Scholar]


