Abstract
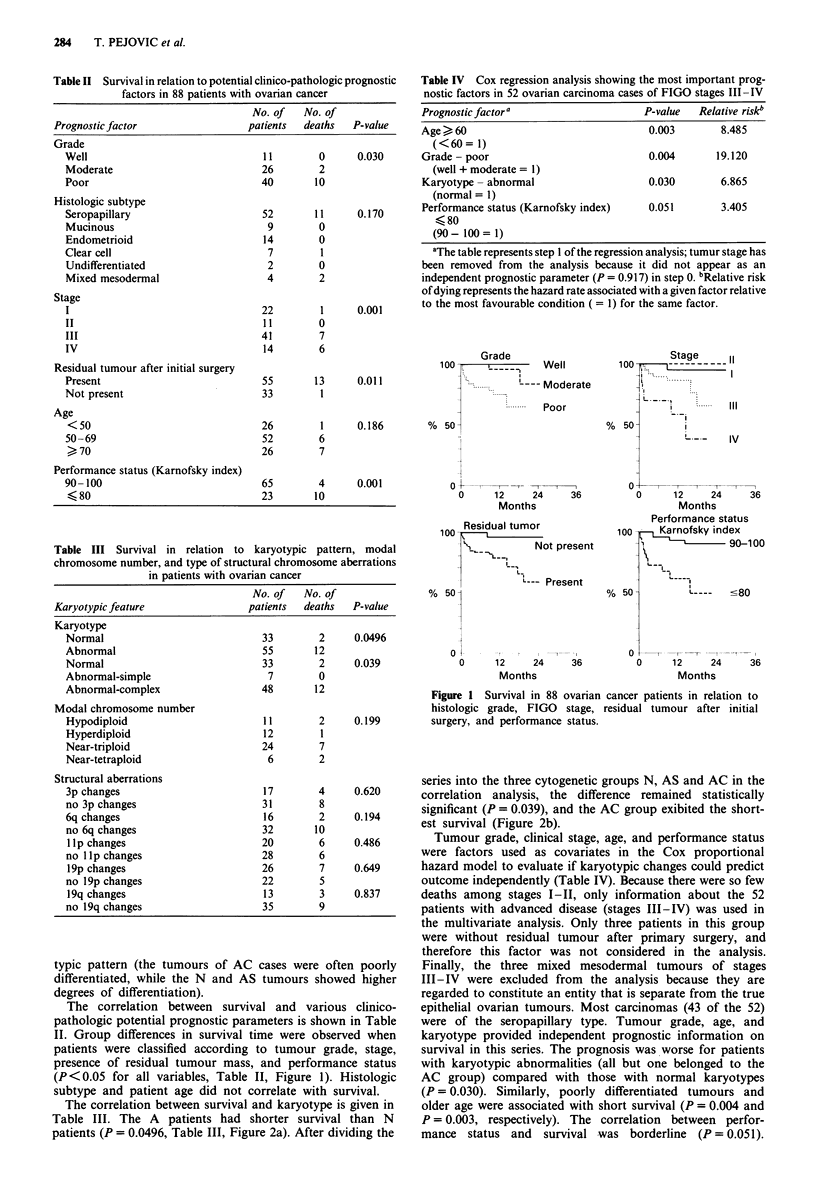
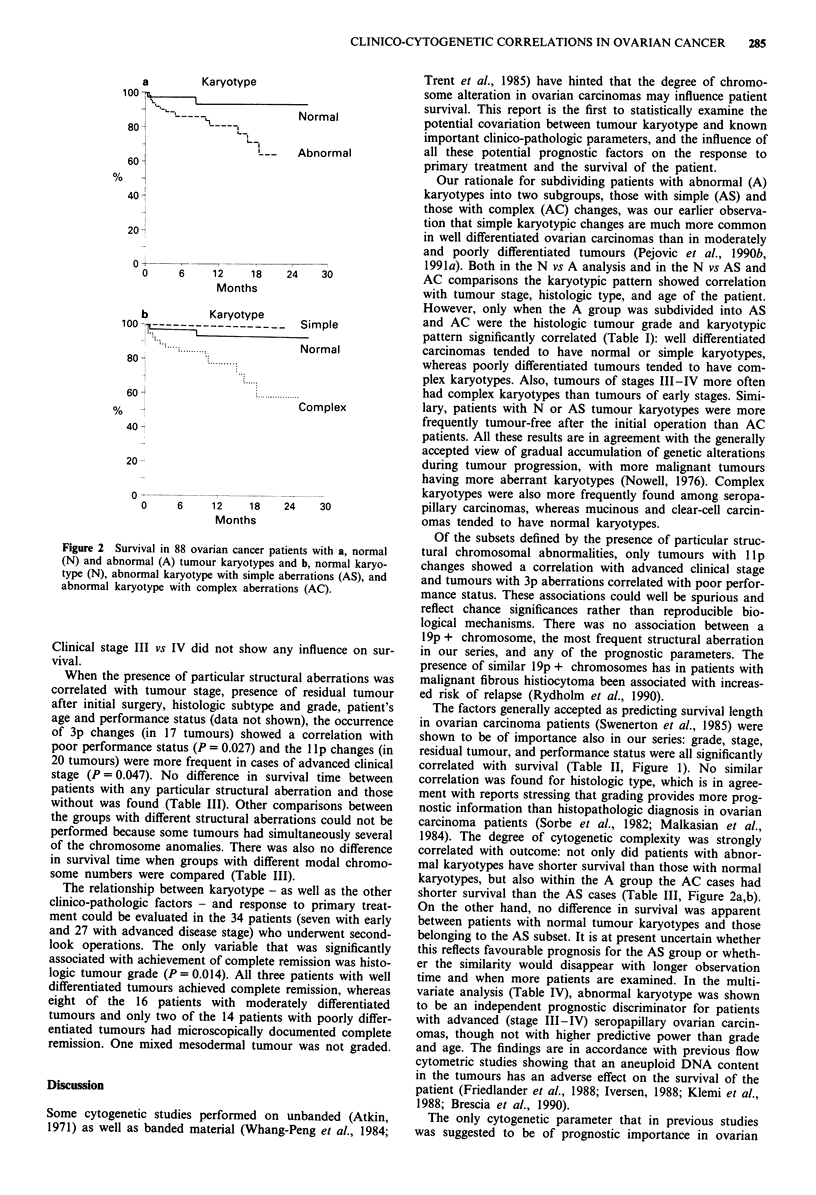
Clinico-cytogenetic correlations were assessed in 88 patients with malignant ovarian tumours. Cytogenetic analysis of the primary tumours yielded normal karyotype (N) in 33 patients and abnormal karyotypes (A) in 55 patients. Within the A group, seven tumours had simple abnormalities (AS), i.e., numerical changes only or a single structural aberration, and 48 had karyotypes with complex aberrations (AC). A correlation analysis between groups N and A revealed that cytogenetic abnormalities were more often found among seropapillary tumours, and that cases with abnormal karyotypes on average were of higher stage and more often had residual tumour mass after initial surgery (P less than 0.05 for all variables). When the three groups N, AS, and AC were compared, they were found to be significantly different with regard not only to the three parameters mentioned above, but now tumour grade also appeared to correlate with karyotypic pattern (P = 0.001), with poorly differentiated tumours having the most complex karyotypes. In a correlation analysis between karyotypic pattern and survival, group A patients had shorter survival than group N (P = 0.049). In the corresponding analysis between groups N, AS, and AC, the differences were also significant (P = 0.039), with shorter survival in group AC than in groups N and AS. Stage, grade, residual tumour after primary surgery, and performance status also correlated with survival time. A multivariate analysis identified abnormal karyotype as being independently associated with short survival in advanced clinical stages (P = 0.030) of ovarian carcinoma. We conclude that cytogenetic analysis of tumour cells may be of clinical value in the assessment of prognosis in patients with malignant ovarian tumours.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur D. C., Berger R., Golomb H. M., Swansbury G. J., Reeves B. R., Alimena G., Van Den Berghe H., Bloomfield C. D., de la Chapelle A., Dewald G. W. The clinical significance of karyotype in acute myelogenous leukemia. Cancer Genet Cytogenet. 1989 Jul 15;40(2):203–216. doi: 10.1016/0165-4608(89)90025-3. [DOI] [PubMed] [Google Scholar]
- Atkin N. B. Modal DNA value and chromosome number in ovarian neoplasia. A clinical and histopathologic assessment. Cancer. 1971 May;27(5):1064–1073. doi: 10.1002/1097-0142(197105)27:5<1064::aid-cncr2820270510>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Bauknecht T., Janz I., Kohler M., Pfleiderer A. Human ovarian carcinomas: correlation of malignancy and survival with the expression of epidermal growth factor receptors (EGF-R) and EGF-like factors (EGF-F). Med Oncol Tumor Pharmacother. 1989;6(2):121–127. doi: 10.1007/BF02985234. [DOI] [PubMed] [Google Scholar]
- Berchuck A., Kamel A., Whitaker R., Kerns B., Olt G., Kinney R., Soper J. T., Dodge R., Clarke-Pearson D. L., Marks P. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990 Jul 1;50(13):4087–4091. [PubMed] [Google Scholar]
- Berchuck A., Rodriguez G. C., Kamel A., Dodge R. K., Soper J. T., Clarke-Pearson D. L., Bast R. C., Jr Epidermal growth factor receptor expression in normal ovarian epithelium and ovarian cancer. I. Correlation of receptor expression with prognostic factors in patients with ovarian cancer. Am J Obstet Gynecol. 1991 Feb;164(2):669–674. doi: 10.1016/s0002-9378(11)80044-x. [DOI] [PubMed] [Google Scholar]
- Bloomfield C. D., Secker-Walker L. M., Goldman A. I., Van Den Berghe H., de la Chapelle A., Ruutu T., Alimena G., Garson O. M., Golomb H. M., Rowley J. D. Six-year follow-up of the clinical significance of karyotype in acute lymphoblastic leukemia. Cancer Genet Cytogenet. 1989 Jul 15;40(2):171–185. doi: 10.1016/0165-4608(89)90023-x. [DOI] [PubMed] [Google Scholar]
- Brescia R. J., Barakat R. A., Beller U., Frederickson G., Suhrland M. J., Dubin N., Demopoulos R. I. The prognostic significance of nuclear DNA content in malignant epithelial tumors of the ovary. Cancer. 1990 Jan 1;65(1):141–147. doi: 10.1002/1097-0142(19900101)65:1<141::aid-cncr2820650128>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Clarke T. J. Histogenesis of ovarian malignant mixed mesodermal tumours. J Clin Pathol. 1990 Apr;43(4):287–290. doi: 10.1136/jcp.43.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich C., Dittrich E., Sevelda P., Hudec M., Salzer H., Grunt T., Eliason J. Clonogenic growth in vitro: an independent biologic prognostic factor in ovarian carcinoma. J Clin Oncol. 1991 Mar;9(3):381–388. doi: 10.1200/JCO.1991.9.3.381. [DOI] [PubMed] [Google Scholar]
- Friedlander M. L., Hedley D. W., Swanson C., Russell P. Prediction of long-term survival by flow cytometric analysis of cellular DNA content in patients with advanced ovarian cancer. J Clin Oncol. 1988 Feb;6(2):282–290. doi: 10.1200/JCO.1988.6.2.282. [DOI] [PubMed] [Google Scholar]
- Iversen O. E. Prognostic value of the flow cytometric DNA index in human ovarian carcinoma. Cancer. 1988 Mar 1;61(5):971–975. doi: 10.1002/1097-0142(19880301)61:5<971::aid-cncr2820610519>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Klemi P. J., Joensuu H., Kiilholma P., Mäenpä J. Clinical significance of abnormal nuclear DNA content in serous ovarian tumors. Cancer. 1988 Nov 1;62(9):2005–2010. doi: 10.1002/1097-0142(19881101)62:9<2005::aid-cncr2820620922>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Malkasian G. D., Jr, Melton L. J., 3rd, O'Brien P. C., Greene M. H. Prognostic significance of histologic classification and grading of epithelial malignancies of the ovary. Am J Obstet Gynecol. 1984 Jun 1;149(3):274–284. doi: 10.1016/0002-9378(84)90227-8. [DOI] [PubMed] [Google Scholar]
- Marsoni S., Torri V., Valsecchi M. G., Belloni C., Bianchi U., Bolis G., Bonazzi C., Colombo N., Epis A., Favalli G. Prognostic factors in advanced epithelial ovarian cancer. (Gruppo Interregionale Cooperativo di Oncologia Ginecologica (GICOG)). Br J Cancer. 1990 Sep;62(3):444–450. doi: 10.1038/bjc.1990.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Pejovic T., Heim S., Mandahl N., Elmfors B., Flodérus U. M., Furgyik S., Helm G., Willén H., Mitelman F. Consistent occurrence of a 19p+ marker chromosome and loss of 11p material in ovarian seropapillary cystadenocarcinomas. Genes Chromosomes Cancer. 1989 Nov;1(2):167–171. doi: 10.1002/gcc.2870010210. [DOI] [PubMed] [Google Scholar]
- Pejovic T., Heim S., Mandahl N., Elmfors B., Furgyik S., Flodérus U. M., Helm G., Willén H., Mitelman F. Bilateral ovarian carcinoma: cytogenetic evidence of unicentric origin. Int J Cancer. 1991 Feb 1;47(3):358–361. doi: 10.1002/ijc.2910470308. [DOI] [PubMed] [Google Scholar]
- Pejovic T., Heim S., Mandahl N., Flodérus U. M., Willén H., Mitelman F. Complex karyotypic anomalies, including an i(5p) marker chromosome, in malignant mixed mesodermal tumor of the ovary. Cancer Genet Cytogenet. 1990 May;46(1):65–69. doi: 10.1016/0165-4608(90)90009-y. [DOI] [PubMed] [Google Scholar]
- Pierre R. V., Catovsky D., Mufti G. J., Swansbury G. J., Mecucci C., Dewald G. W., Ruutu T., Van Den Berghe H., Rowley J. D., Mitelman F. Clinical-cytogenetic correlations in myelodysplasia (preleukemia). Cancer Genet Cytogenet. 1989 Jul 15;40(2):149–161. doi: 10.1016/0165-4608(89)90021-6. [DOI] [PubMed] [Google Scholar]
- Rustin G. J., Gennings J. N., Nelstrop A. E., Covarrubias H., Lambert H. E., Bagshawe K. D. Use of CA-125 to predict survival of patients with ovarian carcinoma. North Thames Cooperative Group. J Clin Oncol. 1989 Nov;7(11):1667–1671. doi: 10.1200/JCO.1989.7.11.1667. [DOI] [PubMed] [Google Scholar]
- Rydholm A., Mandahl N., Heim S., Kreicbergs A., Willén H., Mitelman F. Malignant fibrous histiocytomas with a 19p+ marker chromosome have increased relapse rate. Genes Chromosomes Cancer. 1990 Nov;2(4):296–299. doi: 10.1002/gcc.2870020407. [DOI] [PubMed] [Google Scholar]
- Slotman B. J., Nauta J. J., Rao B. R. Survival of patients with ovarian cancer. Apart from stage and grade, tumor progesterone receptor content is a prognostic indicator. Cancer. 1990 Aug 15;66(4):740–744. doi: 10.1002/1097-0142(19900815)66:4<740::aid-cncr2820660423>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Swenerton K. D., Hislop T. G., Spinelli J., LeRiche J. C., Yang N., Boyes D. A. Ovarian carcinoma: a multivariate analysis of prognostic factors. Obstet Gynecol. 1985 Feb;65(2):264–270. [PubMed] [Google Scholar]
- Trent J. M., Meyskens F. L., Salmon S. E., Ryschon K., Leong S. P., Davis J. R., McGee D. L. Relation of cytogenetic abnormalities and clinical outcome in metastatic melanoma. N Engl J Med. 1990 May 24;322(21):1508–1511. doi: 10.1056/NEJM199005243222107. [DOI] [PubMed] [Google Scholar]
- Volm M., Brüggemann A., Günther M., Kleine W., Pfleiderer A., Vogt-Schaden M. Prognostic relevance of ploidy, proliferation, and resistance-predictive tests in ovarian carcinoma. Cancer Res. 1985 Oct;45(10):5180–5185. [PubMed] [Google Scholar]
- van Houwelingen J. C., ten Bokkel Huinink W. W., van der Burg M. E., van Oosterom A. T., Neijt J. P. Predictability of the survival of patients with advanced ovarian cancer. J Clin Oncol. 1989 Jun;7(6):769–773. doi: 10.1200/JCO.1989.7.6.769. [DOI] [PubMed] [Google Scholar]


