Abstract
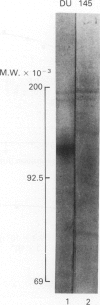
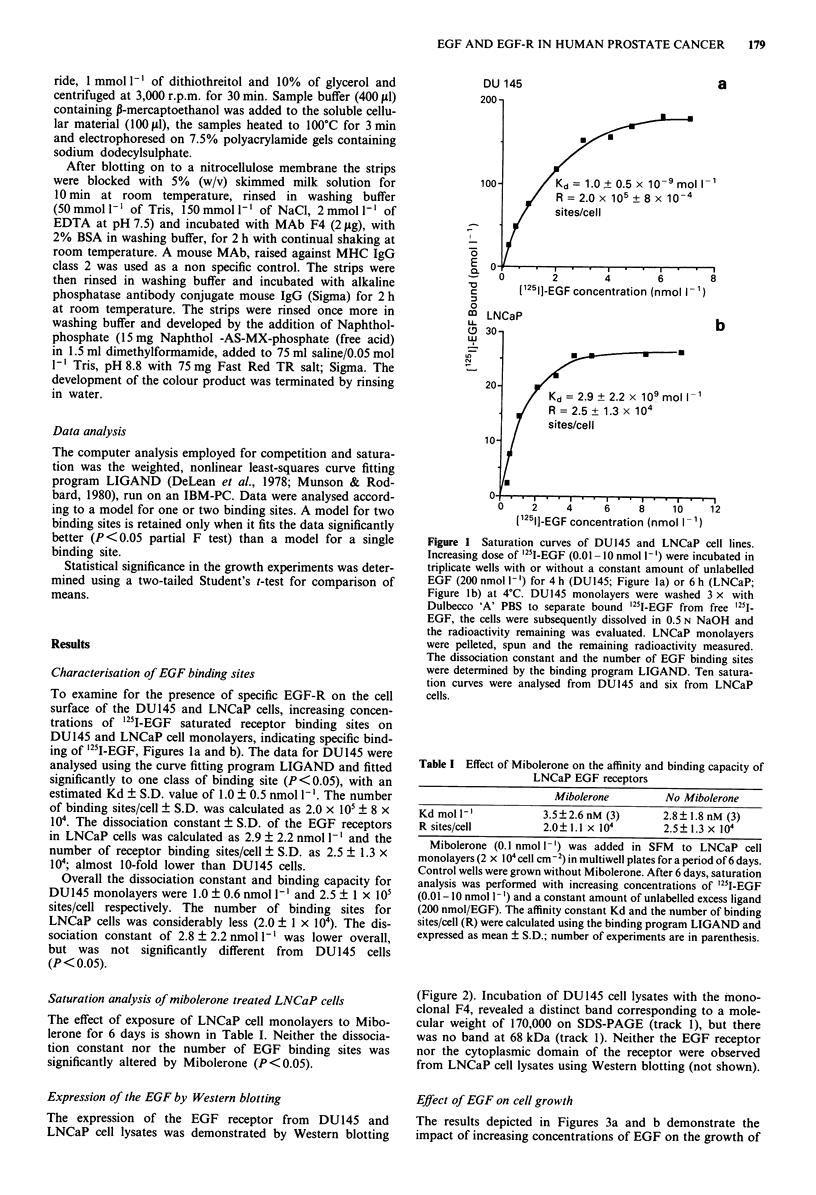
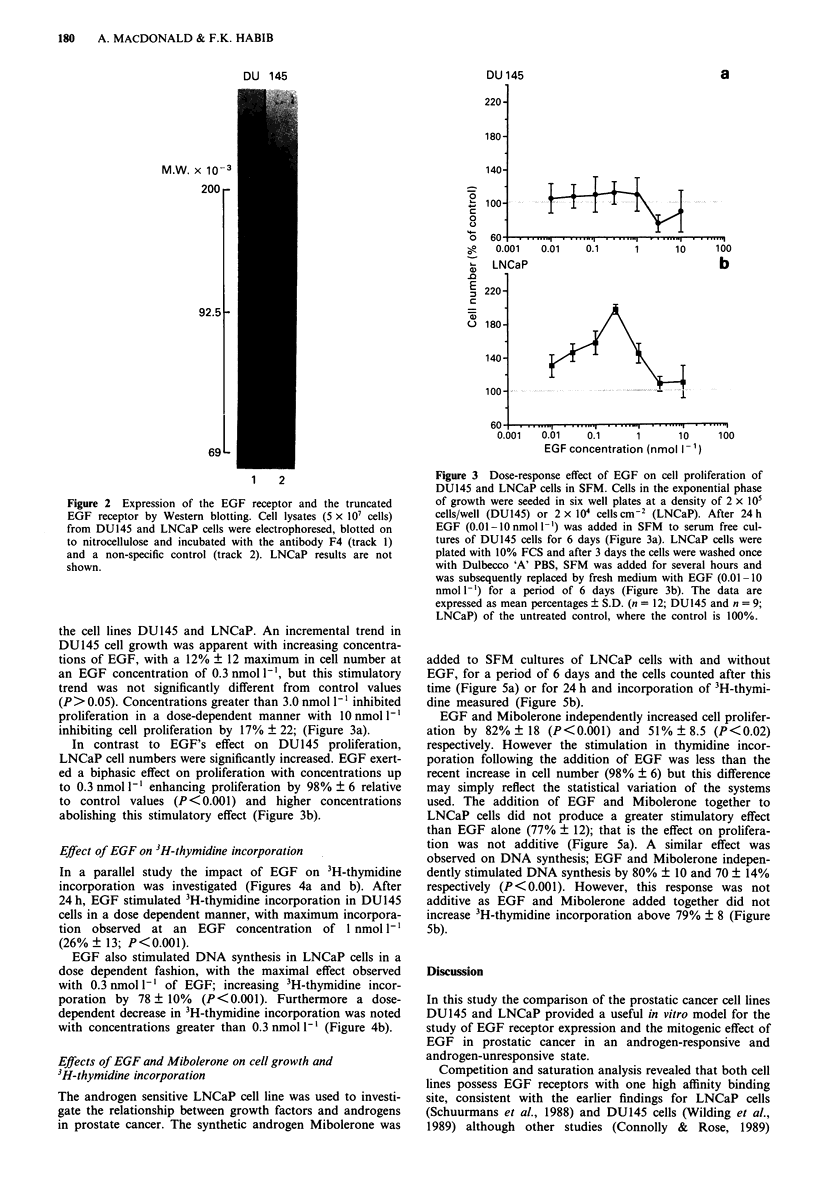
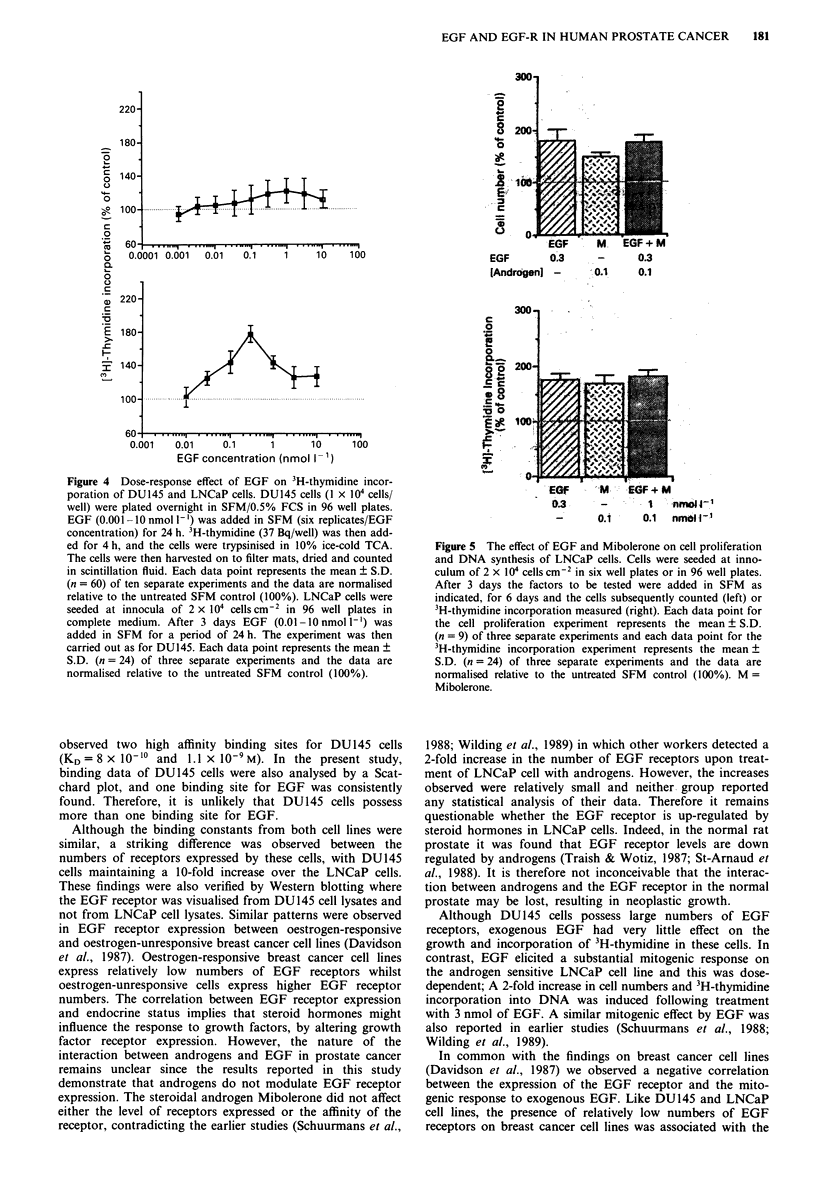
The present study was undertaken to compare the relationship between response to exogenous epidermal growth factor (EGF) and the expression of the EGF-receptor (EGF-R) in an androgen sensitive (LNCaP) and insensitive (DU145) prostate cancer cell line. Although both cell lines demonstrated a single EGF-R binding site of similar high affinities (mean dissociation constant (Kd) +/- S.D. for DU145 = 1.0 +/- 0.6 nmol l-1; LNCaP = 2.8 +/- 2.2 nmol l-1) the number of binding sites (RT) for the hormone insensitive DU145 cells (mean +/- S.D. = 2.5 +/- 1.0 x 10(5) sites/cell) and 10-fold greater than that expressed in the androgen responsive LNCaP cell line (mean +/- S.D. = 2.0 +/- 1 x 10(4) sites/cell). Additionally exogenous EGF only minimally affected the growth and DNA synthesis of DU145 cells whereas LNCaP cells showed a significant response which was dose dependent. The autologous production of EGF-like molecules by DU145 cells is believed to reduce the cells needs for exogenous mitogens, thereby rendering the cells autostimulatory. Treatment of LNCaP cells with Mibolerone--a synthetic androgen--did not affect either the expression of the EGF receptor or the proliferative response observed with EGF. Western blot analysis, using monoclonal antibodies directed against the EGF receptor revealed a band of approximately 170 kD with DU145 cell lysates but the LNCaP EGF receptor was not detected using this technique.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Connolly J. M., Rose D. P. Secretion of epidermal growth factor and related polypeptides by the DU 145 human prostate cancer cell line. Prostate. 1989;15(2):177–186. doi: 10.1002/pros.2990150211. [DOI] [PubMed] [Google Scholar]
- Davidson N. E., Gelmann E. P., Lippman M. E., Dickson R. B. Epidermal growth factor receptor gene expression in estrogen receptor-positive and negative human breast cancer cell lines. Mol Endocrinol. 1987 Mar;1(3):216–223. doi: 10.1210/mend-1-3-216. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Derynck R., Goeddel D. V., Ullrich A., Gutterman J. U., Williams R. D., Bringman T. S., Berger W. H. Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res. 1987 Feb 1;47(3):707–712. [PubMed] [Google Scholar]
- Dickson R. B., Huff K. K., Spencer E. M., Lippman M. E. Induction of epidermal growth factor-related polypeptides by 17 beta-estradiol in MCF-7 human breast cancer cells. Endocrinology. 1986 Jan;118(1):138–142. doi: 10.1210/endo-118-1-138. [DOI] [PubMed] [Google Scholar]
- Griffiths K., Davies P., Eaton C. L., Harper M. E., Peeling W. B., Turkes A. O., Turkes A., Wilson D. W., Pierrepoint C. G. Cancer of the prostate: endocrine factors. Oxf Rev Reprod Biol. 1987;9:192–259. [PubMed] [Google Scholar]
- Gullick W. J., Marsden J. J., Whittle N., Ward B., Bobrow L., Waterfield M. D. Expression of epidermal growth factor receptors on human cervical, ovarian, and vulval carcinomas. Cancer Res. 1986 Jan;46(1):285–292. [PubMed] [Google Scholar]
- Horoszewicz J. S., Leong S. S., Kawinski E., Karr J. P., Rosenthal H., Chu T. M., Mirand E. A., Murphy G. P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983 Apr;43(4):1809–1818. [PubMed] [Google Scholar]
- King R. J. Receptors, growth factors and steroid insensitivity of tumours. J Endocrinol. 1990 Feb;124(2):179–181. doi: 10.1677/joe.0.1240179. [DOI] [PubMed] [Google Scholar]
- Knabbe C., Lippman M. E., Wakefield L. M., Flanders K. C., Kasid A., Derynck R., Dickson R. B. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987 Feb 13;48(3):417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- MacDonald A., Chisholm G. D., Habib F. K. Production and response of a human prostatic cancer line to transforming growth factor-like molecules. Br J Cancer. 1990 Oct;62(4):579–584. doi: 10.1038/bjc.1990.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Murphy L. J., Sutherland R. L., Stead B., Murphy L. C., Lazarus L. Progestin regulation of epidermal growth factor receptor in human mammary carcinoma cells. Cancer Res. 1986 Feb;46(2):728–734. [PubMed] [Google Scholar]
- Schuurmans A. L., Bolt J., Mulder E. Androgens stimulate both growth rate and epidermal growth factor receptor activity of the human prostate tumor cell LNCaP. Prostate. 1988;12(1):55–63. doi: 10.1002/pros.2990120108. [DOI] [PubMed] [Google Scholar]
- St-Arnaud R., Poyet P., Walker P., Labrie F. Androgens modulate epidermal growth factor receptor levels in the rat ventral prostate. Mol Cell Endocrinol. 1988 Mar;56(1-2):21–27. doi: 10.1016/0303-7207(88)90004-4. [DOI] [PubMed] [Google Scholar]
- Traish A. M., Wotiz H. H. Prostatic epidermal growth factor receptors and their regulation by androgens. Endocrinology. 1987 Oct;121(4):1461–1467. doi: 10.1210/endo-121-4-1461. [DOI] [PubMed] [Google Scholar]
- Wilding G., Valverius E., Knabbe C., Gelmann E. P. Role of transforming growth factor-alpha in human prostate cancer cell growth. Prostate. 1989;15(1):1–12. doi: 10.1002/pros.2990150102. [DOI] [PubMed] [Google Scholar]