Abstract
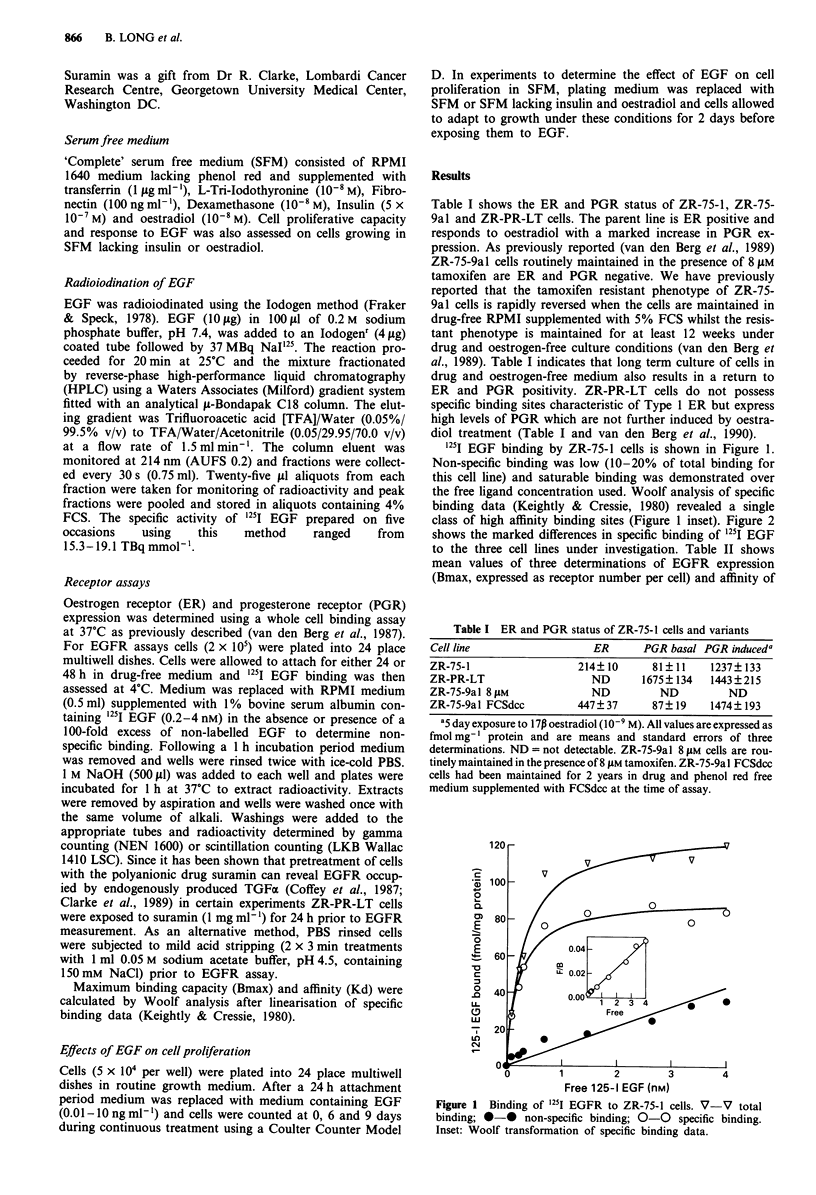
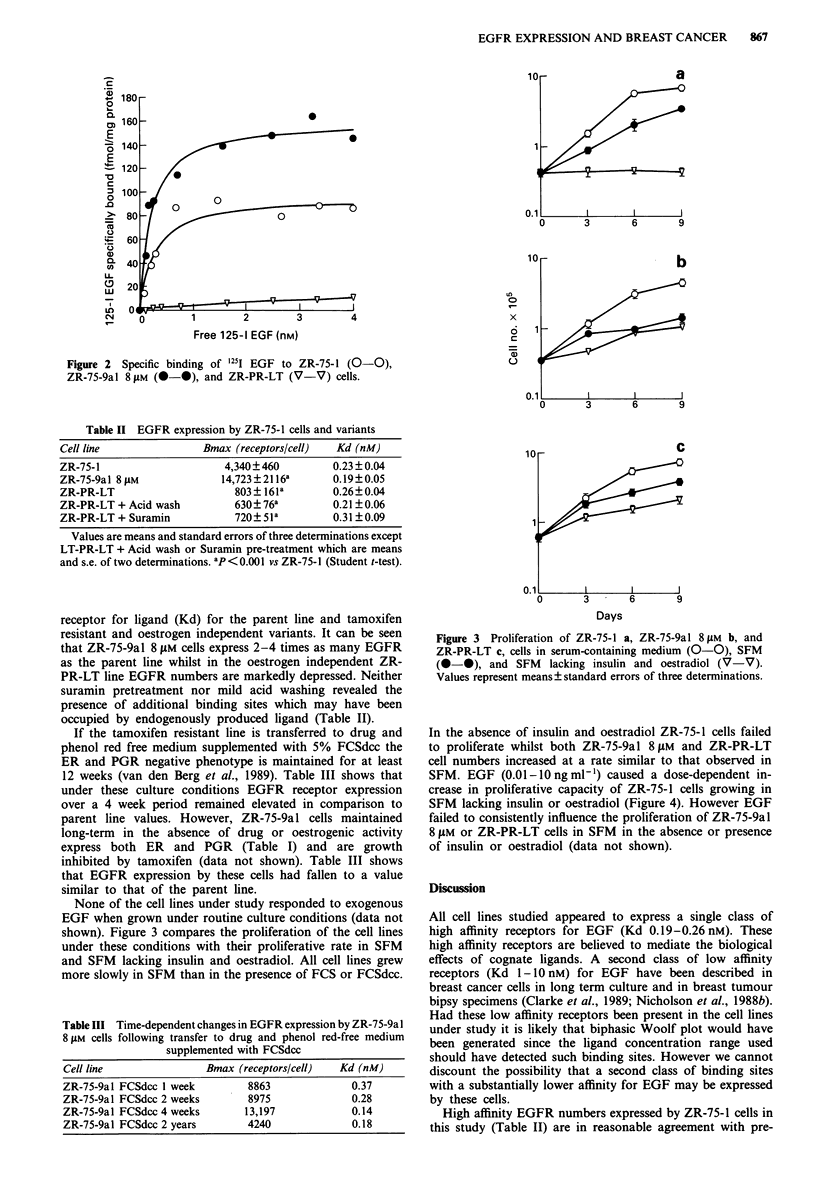
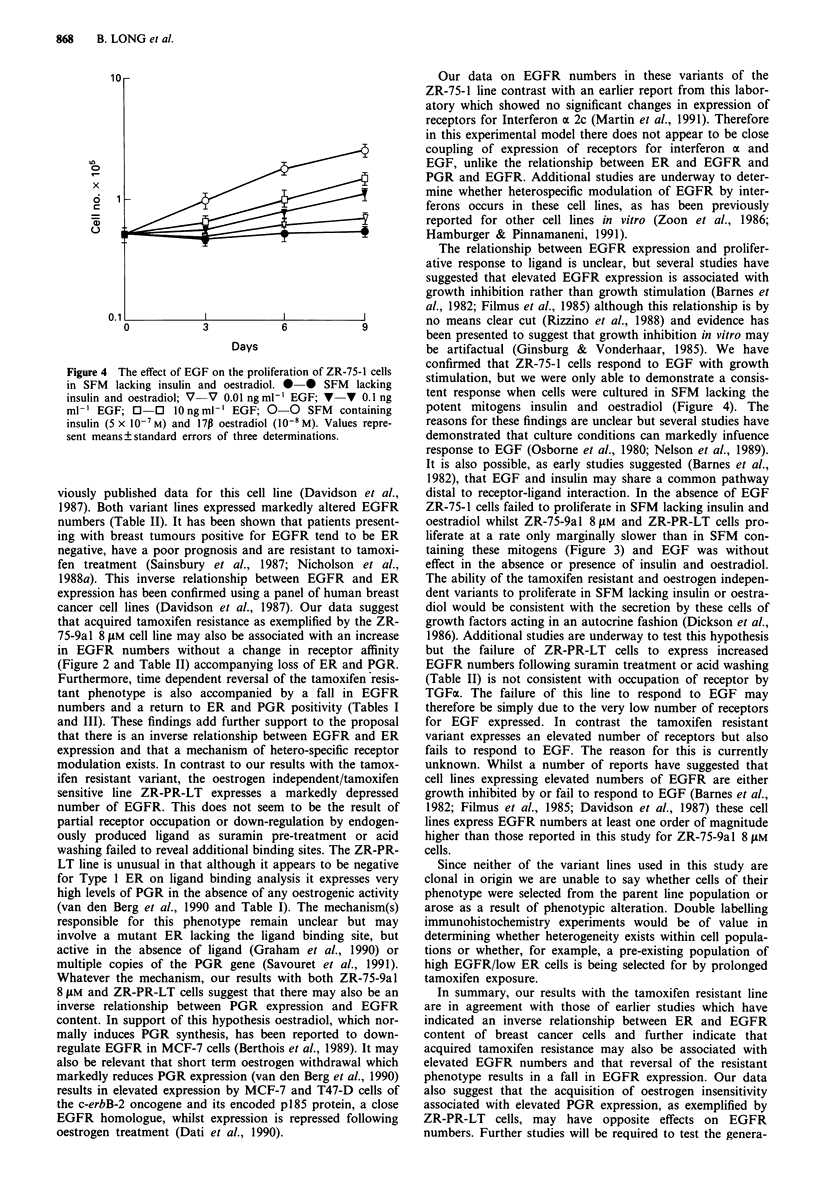
We have examined the expression of receptors for epidermal growth factor (EGFR) by the ZR-75-1 human breast cancer cell line and tamoxifen resistant (ZR-75-9al 8 microM) and oestrogen independent/tamoxifen sensitive (ZR-PR-LT) variants. The parent line expressed a single class of high affinity binding sites (4,340 +/- 460 receptors/cell; Kd 0.23 +/- 0.04 nM). ZR-75-9al 8 microM cells, routinely maintained in medium containing 8 microM tamoxifen, were negative for oestrogen receptor (ER) and progesterone receptor (PGR) and expressed a markedly increased number of EGFR (14,723 +/- 2116 receptors/cell). Receptor affinity was unchanged. Time dependent reversal of the tamoxifen resistant phenotype was accompanied by a return to ER and PGR positivity and a fall in EGFR numbers to parent cell levels. In contrast ZR-PR-LT cells had a greatly reduced EGFR content (803 +/- 161 receptors/cell) accompanying elevated PGR numbers. Pre-treatment of these cells with suramin or mild acid stripping failed to expose receptors which may have been occupied by endogenously produced ligand. Increased proliferation of ZR-75-1 cells treated with EGFR (0.01-10 ng ml-1) was only observed in serum-free medium lacking insulin and oestradiol. Under these conditions untreated cells failed to proliferate. Both variant lines continued to proliferate in serum free medium in the absence or presence of insulin and oestradiol but failed to respond to exogenous EGF.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D. W. Epidermal growth factor inhibits growth of A431 human epidermoid carcinoma in serum-free cell culture. J Cell Biol. 1982 Apr;93(1):1–4. doi: 10.1083/jcb.93.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthois Y., Dong X. F., Martin P. M. Regulation of epidermal growth factor-receptor by estrogen and antiestrogen in the human breast cancer cell line MCF-7. Biochem Biophys Res Commun. 1989 Feb 28;159(1):126–131. doi: 10.1016/0006-291x(89)92413-3. [DOI] [PubMed] [Google Scholar]
- Clarke R., Brünner N., Katz D., Glanz P., Dickson R. B., Lippman M. E., Kern F. G. The effects of a constitutive expression of transforming growth factor-alpha on the growth of MCF-7 human breast cancer cells in vitro and in vivo. Mol Endocrinol. 1989 Feb;3(2):372–380. doi: 10.1210/mend-3-2-372. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Leof E. B., Shipley G. D., Moses H. L. Suramin inhibition of growth factor receptor binding and mitogenicity in AKR-2B cells. J Cell Physiol. 1987 Jul;132(1):143–148. doi: 10.1002/jcp.1041320120. [DOI] [PubMed] [Google Scholar]
- Dati C., Antoniotti S., Taverna D., Perroteau I., De Bortoli M. Inhibition of c-erbB-2 oncogene expression by estrogens in human breast cancer cells. Oncogene. 1990 Jul;5(7):1001–1006. [PubMed] [Google Scholar]
- Davidson N. E., Gelmann E. P., Lippman M. E., Dickson R. B. Epidermal growth factor receptor gene expression in estrogen receptor-positive and negative human breast cancer cell lines. Mol Endocrinol. 1987 Mar;1(3):216–223. doi: 10.1210/mend-1-3-216. [DOI] [PubMed] [Google Scholar]
- Derynck R. Transforming growth factor alpha. Cell. 1988 Aug 26;54(5):593–595. doi: 10.1016/s0092-8674(88)80001-1. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., Bates S. E., McManaway M. E., Lippman M. E. Characterization of estrogen responsive transforming activity in human breast cancer cell lines. Cancer Res. 1986 Apr;46(4 Pt 1):1707–1713. [PubMed] [Google Scholar]
- Filmus J., Pollak M. N., Cailleau R., Buick R. N. MDA-468, a human breast cancer cell line with a high number of epidermal growth factor (EGF) receptors, has an amplified EGF receptor gene and is growth inhibited by EGF. Biochem Biophys Res Commun. 1985 Apr 30;128(2):898–905. doi: 10.1016/0006-291x(85)90131-7. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Ginsburg E., Vonderhaar B. K. Epidermal growth factor stimulates the growth of A431 tumors in athymic mice. Cancer Lett. 1985 Sep 15;28(2):143–150. doi: 10.1016/0304-3835(85)90069-2. [DOI] [PubMed] [Google Scholar]
- Graham M. L., 2nd, Krett N. L., Miller L. A., Leslie K. K., Gordon D. F., Wood W. M., Wei L. L., Horwitz K. B. T47DCO cells, genetically unstable and containing estrogen receptor mutations, are a model for the progression of breast cancers to hormone resistance. Cancer Res. 1990 Oct 1;50(19):6208–6217. [PubMed] [Google Scholar]
- Hamburger A. W., Pinnamaneni G. D. Increased epidermal growth factor receptor gene expression by gamma-interferon in a human breast carcinoma cell line. Br J Cancer. 1991 Jul;64(1):64–68. doi: 10.1038/bjc.1991.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley D. D., Cressie N. A. The Woolf plot is more reliable than the Scatchard plot in analysing data from hormone receptor assays. J Steroid Biochem. 1980 Nov;13(11):1317–1323. doi: 10.1016/0022-4731(80)90092-8. [DOI] [PubMed] [Google Scholar]
- Knabbe C., Lippman M. E., Wakefield L. M., Flanders K. C., Kasid A., Derynck R., Dickson R. B. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987 Feb 13;48(3):417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Lippman M. E., Dickson R. B., Gelmann E. P., Rosen N., Knabbe C., Bates S., Bronzert D., Huff K., Kasid A. Growth regulation of human breast carcinoma occurs through regulated growth factor secretion. J Cell Biochem. 1987 Sep;35(1):1–16. doi: 10.1002/jcb.240350102. [DOI] [PubMed] [Google Scholar]
- Martin J. H., McKibben B. M., Lynch M., van den Berg H. W. Modulation by oestrogen and progestins/antiprogestins of alpha interferon receptor expression in human breast cancer cells. Eur J Cancer. 1991;27(2):143–146. doi: 10.1016/0277-5379(91)90473-q. [DOI] [PubMed] [Google Scholar]
- Murphy L. J., Sutherland R. L., Lazarus L. Regulation of growth hormone and epidermal growth factor receptors by progestins in breast cancer cells. Biochem Biophys Res Commun. 1985 Sep 16;131(2):767–773. doi: 10.1016/0006-291x(85)91305-1. [DOI] [PubMed] [Google Scholar]
- Nelson J., McGivern M., Walker B., Bailie J. R., Murphy R. F. Growth-inhibitory and growth-stimulatory effects of epidermal growth factor on human breast cancer cell line, MDA.MB.436: dependence on culture conditions. Eur J Cancer Clin Oncol. 1989 Dec;25(12):1851–1855. doi: 10.1016/0277-5379(89)90358-1. [DOI] [PubMed] [Google Scholar]
- Nicholson S., Halcrow P., Sainsbury J. R., Angus B., Chambers P., Farndon J. R., Harris A. L. Epidermal growth factor receptor (EGFr) status associated with failure of primary endocrine therapy in elderly postmenopausal patients with breast cancer. Br J Cancer. 1988 Dec;58(6):810–814. doi: 10.1038/bjc.1988.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson S., Sainsbury J. R., Needham G. K., Chambers P., Farndon J. R., Harris A. L. Quantitative assays of epidermal growth factor receptor in human breast cancer: cut-off points of clinical relevance. Int J Cancer. 1988 Jul 15;42(1):36–41. doi: 10.1002/ijc.2910420108. [DOI] [PubMed] [Google Scholar]
- Osborne C. K., Hamilton B., Titus G., Livingston R. B. Epidermal growth factor stimulation of human breast cancer cells in culture. Cancer Res. 1980 Jul;40(7):2361–2366. [PubMed] [Google Scholar]
- Rizzino A., Ruff E., Kazakoff P. Isolation and characterization of A-431 cells that retain high epidermal growth factor binding capacity and respond to epidermal growth factor by growth stimulation. Cancer Res. 1988 May 1;48(9):2377–2381. [PubMed] [Google Scholar]
- Sainsbury J. R., Farndon J. R., Needham G. K., Malcolm A. J., Harris A. L. Epidermal-growth-factor receptor status as predictor of early recurrence of and death from breast cancer. Lancet. 1987 Jun 20;1(8547):1398–1402. doi: 10.1016/s0140-6736(87)90593-9. [DOI] [PubMed] [Google Scholar]
- Savouret J. F., Fridlanski F., Atger M., Misrahi M., Berger R., Milgrom E. Origin of the high constitutive level of progesterone receptor in T47-D breast cancer cells. Mol Cell Endocrinol. 1991 Feb;75(2):157–162. doi: 10.1016/0303-7207(91)90230-p. [DOI] [PubMed] [Google Scholar]
- Zoon K. C., Karasaki Y., zur Nedden D. L., Hu R. Q., Arnheiter H. Modulation of epidermal growth factor receptors by human alpha interferon. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8226–8230. doi: 10.1073/pnas.83.21.8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg H. W., Leahey W. J., Lynch M., Clarke R., Nelson J. Recombinant human interferon alpha increases oestrogen receptor expression in human breast cancer cells (ZR-75-1) and sensitizes them to the anti-proliferative effects of tamoxifen. Br J Cancer. 1987 Mar;55(3):255–257. doi: 10.1038/bjc.1987.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg H. W., Lynch M., Martin J., Nelson J., Dickson G. R., Crockard A. D. Characterisation of a tamoxifen-resistant variant of the ZR-75-1 human breast cancer cell line (ZR-75-9a1) and ability of the resistant phenotype. Br J Cancer. 1989 Apr;59(4):522–526. doi: 10.1038/bjc.1989.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg H. W., Martin J., Lynch M. High progesterone receptor concentration in a variant of the ZR-75-1 human breast cancer cell line adapted to growth in oestrogen free conditions. Br J Cancer. 1990 Apr;61(4):504–507. doi: 10.1038/bjc.1990.114. [DOI] [PMC free article] [PubMed] [Google Scholar]


