Abstract
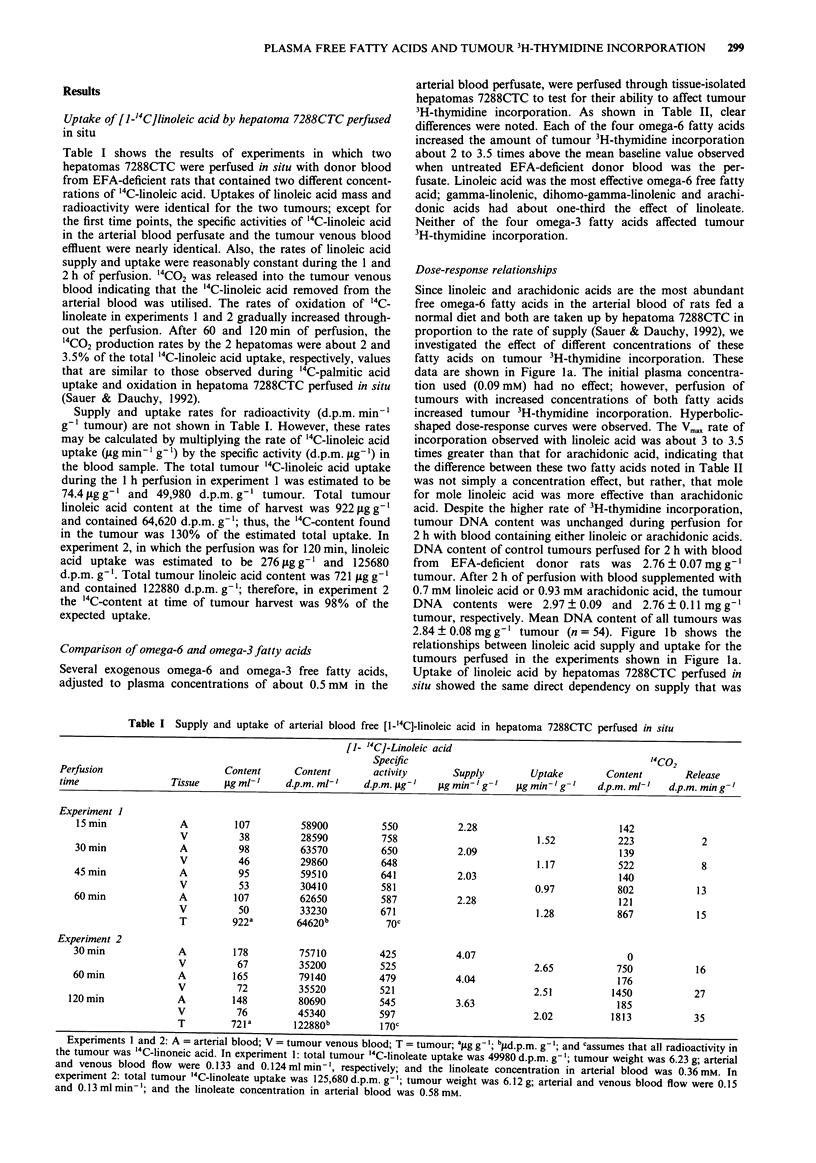
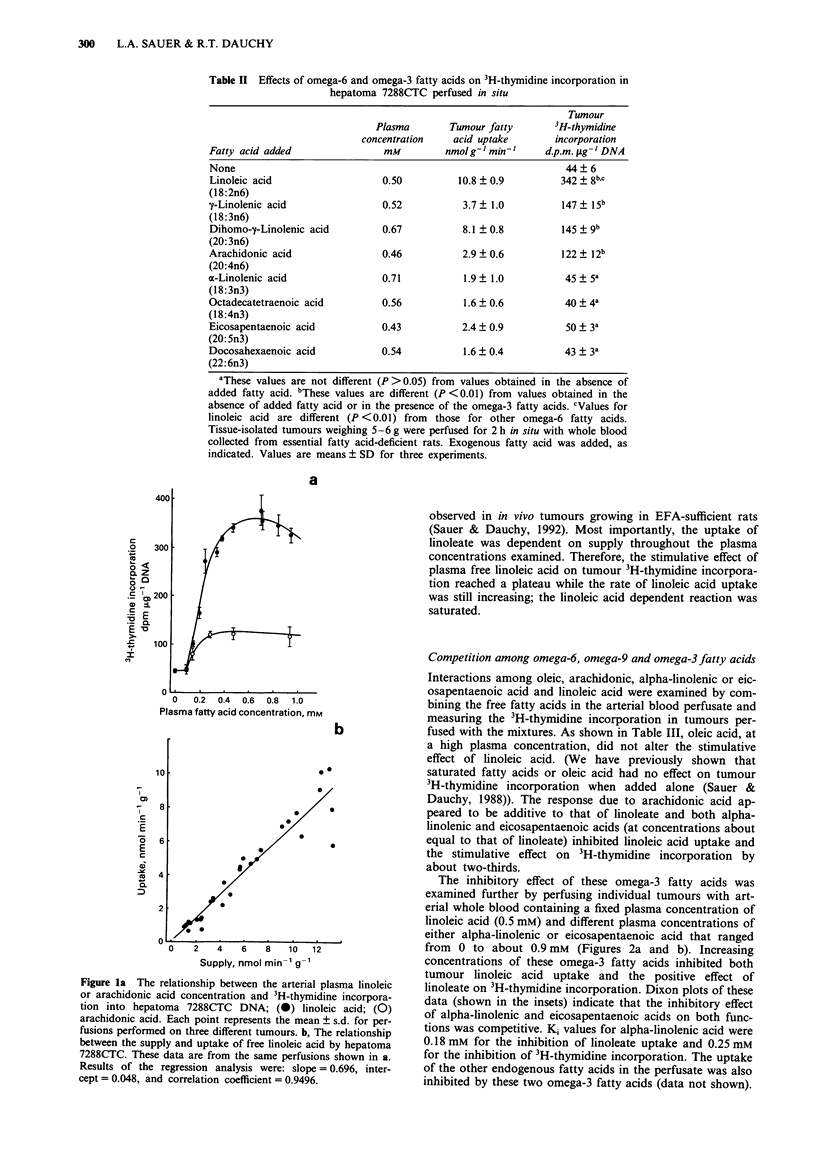
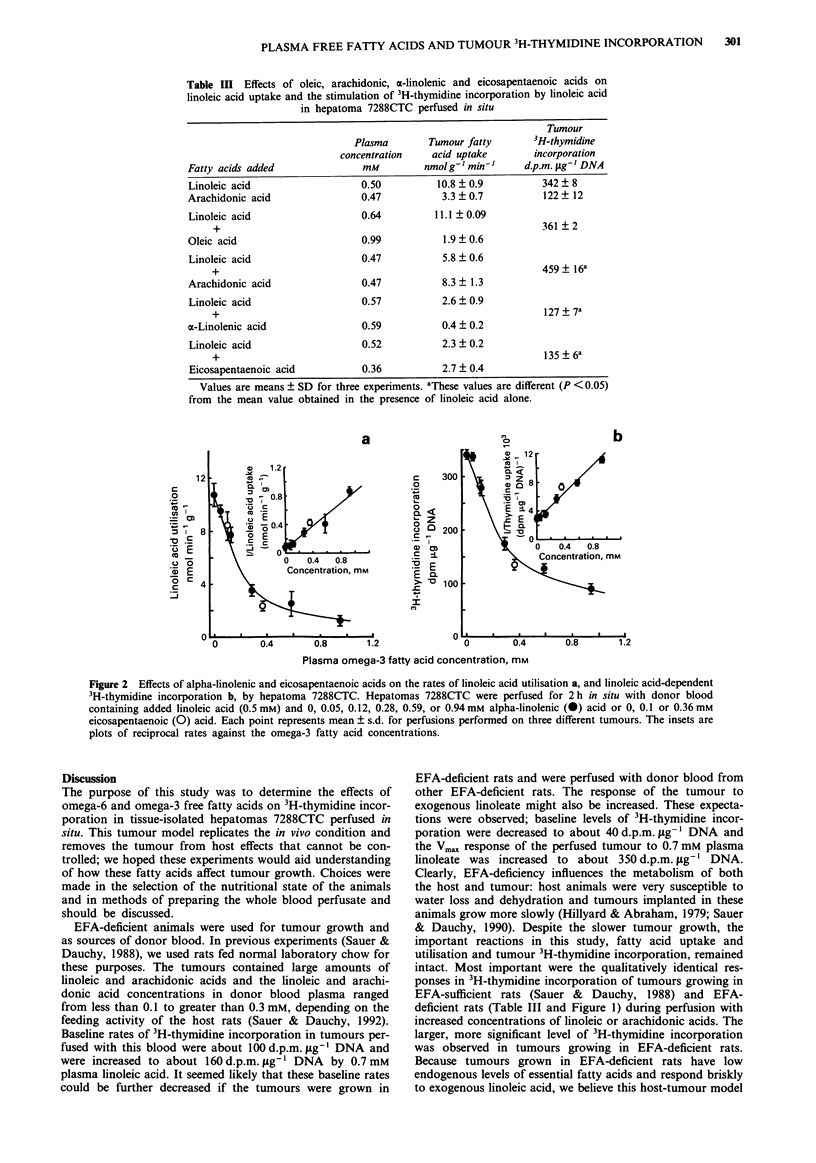
Ingestion of diets containing corn oil or marine fish oils is known to increase or decrease, respectively, the growth of transplantable rodent tumours. The active agents in these oils have been identified as linoleic acid (in corn oil) and omega-3 fatty acids (in marine oils), but it is still not known how they influence the tumour growth processes. In these experiments we examined the effects of plasma free omega-6 and omega-3 fatty acids on the rate of 3H-thymidine incorporation in tissue-isolated hepatoma 7288CTC perfused in situ. Host Buffalo rats were fed an essential fatty acid-deficient diet. Plasma and tumours in these animals contained low endogenous levels of both omega-6 and omega-3 fatty acids. Perfusion of these tumours for 2 h with donor whole blood containing added omega-6 free fatty acids, including 0.5 mM linoleic (C18:2,N-6), gamma-linolenic (C18:3,N-6), dihomo-gamma-linolenic (C20:3,N-6) or arachidonic acids (C20:4,N-6), increased the rate of 3H-thymidine incorporation. Linoleic acid was about three times more effective than the other omega-6 fatty acids. Typical hyperbolic substrate-saturation curves were observed as the plasma free linoleate or arachidonate concentration was increased. When perfused alone plasma free omega-3 fatty acids had no effect on tumour 3H-thymidine incorporation, but in the presence of linoleic acid the omega-3 fatty acids, alpha-linolenic (C18:3,N-3) and eicosapentaenoic (C20:5,N-3), competitively inhibited both tumour linoleate uptake and the stimulative effect on 3H-thymidine incorporation. The results suggest that the ambient plasma free linoleic and arachidonic acid concentrations in host arterial blood directly influence the rate of tumour DNA synthesis. Plasma free omega-3 fatty acids appear to modulate the effect of linoleic acid by competitively inhibiting its uptake. These relationships could explain the actions of dietary linoleic and omega-3 fatty acids on tumour growth in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S., Hillyard L. A. Effect of dietary 18-carbon fatty acids on growth of transplantable mammary adenocarcinomas in mice. J Natl Cancer Inst. 1983 Sep;71(3):601–605. [PubMed] [Google Scholar]
- Gabor H., Abraham S. Effect of dietary menhaden oil on tumor cell loss and the accumulation of mass of a transplantable mammary adenocarcinoma in BALB/c mice. J Natl Cancer Inst. 1986 Jun;76(6):1223–1229. [PubMed] [Google Scholar]
- Gabor H., Hillyard L. A., Abraham S. Effect of dietary fat on growth kinetics of transplantable mammary adenocarcinoma in BALB/c mice. J Natl Cancer Inst. 1985 Jun;74(6):1299–1305. [PubMed] [Google Scholar]
- HOLMAN R. T. The ratio of trienoic: tetraenoic acids in tissue lipids as a measure of essential fatty acid requirement. J Nutr. 1960 Mar;70:405–410. doi: 10.1093/jn/70.3.405. [DOI] [PubMed] [Google Scholar]
- Hillyard L. A., Abraham S. Effect of dietary polyunsaturated fatty acids on growth of mammary adenocarcinomas in mice and rats. Cancer Res. 1979 Nov;39(11):4430–4437. [PubMed] [Google Scholar]
- Hillyard L., Rao G. A., Abraham S. Effect of dietary fat on fatty acid composition of mouse and rat mammary adenocarcinomas. Proc Soc Exp Biol Med. 1980 Mar;163(3):376–383. doi: 10.3181/00379727-163-40781. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Baldwin J. H., Kiernan J. A. Control of growth of a tumor cell by linoleic acid. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3976–3978. doi: 10.1073/pnas.71.10.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip C., Carter C. A., Ip M. M. Requirement of essential fatty acid for mammary tumorigenesis in the rat. Cancer Res. 1985 May;45(5):1997–2001. [PubMed] [Google Scholar]
- Jurkowski J. J., Cave W. T., Jr Dietary effects of menhaden oil on the growth and membrane lipid composition of rat mammary tumors. J Natl Cancer Inst. 1985 May;74(5):1145–1150. [PubMed] [Google Scholar]
- Karmali R. A., Marsh J., Fuchs C. Effect of omega-3 fatty acids on growth of a rat mammary tumor. J Natl Cancer Inst. 1984 Aug;73(2):457–461. doi: 10.1093/jnci/73.2.457. [DOI] [PubMed] [Google Scholar]
- NESTEL P. J., STEINBERG D. FATE OF PALMITATE AND OF LINOLEATE PERFUSED THROUGH THE ISOLATED RAT LIVER AT HIGH CONCENTRATIONS. J Lipid Res. 1963 Oct;4:461–469. [PubMed] [Google Scholar]
- Rogers A. E., Wetsel W. C. Mammary carcinogenesis in rats fed different amounts and types of fat. Cancer Res. 1981 Sep;41(9 Pt 2):3735–3737. [PubMed] [Google Scholar]
- Rose D. P., Connolly J. M. Effects of fatty acids and inhibitors of eicosanoid synthesis on the growth of a human breast cancer cell line in culture. Cancer Res. 1990 Nov 15;50(22):7139–7144. [PubMed] [Google Scholar]
- Sauer L. A., Dauchy R. T. Identification of linoleic and arachidonic acids as the factors in hyperlipemic blood that increase [3H]thymidine incorporation in hepatoma 7288CTC perfused in situ. Cancer Res. 1988 Jun 1;48(11):3106–3111. [PubMed] [Google Scholar]
- Sauer L. A., Dauchy R. T. Tumour-host metabolic interrelationships. Biochem Soc Trans. 1990 Feb;18(1):80–82. doi: 10.1042/bst0180080. [DOI] [PubMed] [Google Scholar]
- Sauer L. A., Dauchy R. T. Uptake of plasma lipids by tissue-isolated hepatomas 7288CTC and 7777 in vivo. Br J Cancer. 1992 Aug;66(2):290–296. doi: 10.1038/bjc.1992.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer L. A., Nagel W. O., Dauchy R. T., Miceli L. A., Austin J. E. Stimulation of tumor growth in adult rats in vivo during an acute fast. Cancer Res. 1986 Jul;46(7):3469–3475. [PubMed] [Google Scholar]
- Sauer L. A., Stayman J. W., 3rd, Dauchy R. T. Amino acid, glucose, and lactic acid utilization in vivo by rat tumors. Cancer Res. 1982 Oct;42(10):4090–4097. [PubMed] [Google Scholar]
- Soler-Argilaga C., Infante R., Renaud G., Polonovski J. Factors influencing free fatty acid uptake by the isolated perfused rat liver. Biochimie. 1974;56(5):757–761. doi: 10.1016/s0300-9084(74)80047-7. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Hoak J. C. An improved method for the addition of long-chain free fatty acid to protein solutions. Anal Biochem. 1969 Nov;32(2):297–302. doi: 10.1016/0003-2697(69)90089-x. [DOI] [PubMed] [Google Scholar]
- Travis J., Pannell R. Selective removal of albumin from plasma by affinity chromatography. Clin Chim Acta. 1973 Nov 23;49(1):49–52. doi: 10.1016/0009-8981(73)90341-0. [DOI] [PubMed] [Google Scholar]
- Van Harken D. R., Dixon C. W., Heimberg M. Hepatic lipid metabolism in experimental diabetes. V. The effect of concentration of oleate on metabolism of triglycerides and on ketogenesis. J Biol Chem. 1969 May 10;244(9):2278–2285. [PubMed] [Google Scholar]
- Welsch C. W. Enhancement of mammary tumorigenesis by dietary fat: review of potential mechanisms. Am J Clin Nutr. 1987 Jan;45(1 Suppl):192–202. doi: 10.1093/ajcn/45.1.192. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Kidwell W. R. Effects of free fatty acids on the growth of normal and neoplastic rat mammary epithelial cells. Cancer Res. 1979 Feb;39(2 Pt 1):426–435. [PubMed] [Google Scholar]


