Abstract
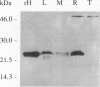
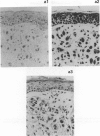
The tissue and cellular distribution of the DNA repair protein O6-alkylguanine-DNA-alkyltransferase (ATase) is an important question in relation to the response of tumour and normal tissues to chemotherapeutic regimes employing alkylating agents such as methyltriazenes and nitrosoureas. In order to examine this issue by immunostaining, we have raised a rabbit antiserum to apparently pure recombinant human enzyme. The antiserum is highly specific and sensitive, detecting a band at 24 kDa on western blots of crude extracts of ATase-expressing human lymphoblastoid cells, liver and melanoma. Adjacent sections of acetone or formalin fixed normal human liver and subcutaneous malignant melanoma were reacted with preimmune serum or antiserum and an immunoperoxidase detection system with silver enhancement was used to locate binding of the primary antibody to the antigen. In sections reacted with preimmune serum or with antigen-preadsorbed antiserum, only faint cytoplasmic and little or no nuclear staining was seen. In contrast, using antiserum, the reaction in positively staining cells was very intense and predominantly nuclear. In the liver, there was interindividual variation in the cellular distribution of reaction with staining present in all discernable cell types in most samples but confined to the hepatocytes and bile duct epithelial cells in others. In the melanoma sections, all discernable cell types showed mainly nuclear staining: the intensity of staining varied between tissue samples and there was evidence of a range of intermediate staining intensities with some melanoma cells showing no detectable reaction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartsch H., Montesano R. Relevance of nitrosamines to human cancer. Carcinogenesis. 1984 Nov;5(11):1381–1393. doi: 10.1093/carcin/5.11.1381. [DOI] [PubMed] [Google Scholar]
- D'Incalci M., Citti L., Taverna P., Catapano C. V. Importance of the DNA repair enzyme O6-alkyl guanine alkyltransferase (AT) in cancer chemotherapy. Cancer Treat Rev. 1988 Dec;15(4):279–292. doi: 10.1016/0305-7372(88)90026-6. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Babich M. A., Yarosh D. B., Scudiero D. A. The role of O6-methylguanine in human cell killing, sister chromatid exchange induction and mutagenesis: a review. J Cell Sci Suppl. 1987;6:333–353. doi: 10.1242/jcs.1984.supplement_6.22. [DOI] [PubMed] [Google Scholar]
- Fan C. Y., Potter P. M., Rafferty J., Watson A. J., Cawkwell L., Searle P. F., O'Connor P. J., Margison G. P. Expression of a human O6-alkylguanine-DNA-alkyltransferase cDNA in human cells and transgenic mice. Nucleic Acids Res. 1990 Oct 11;18(19):5723–5727. doi: 10.1093/nar/18.19.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson S. L. Regeneration of O6-alkylguanine-DNA alkyltransferase in human lymphocytes after nitrosourea exposure. Cancer Res. 1988 Sep 15;48(18):5368–5373. [PubMed] [Google Scholar]
- Gerson S. L., Trey J. E., Miller K. Potentiation of nitrosourea cytotoxicity in human leukemic cells by inactivation of O6-alkylguanine-DNA alkyltransferase. Cancer Res. 1988 Mar 15;48(6):1521–1527. [PubMed] [Google Scholar]
- Hayward I. P., Parsons P. G. Comparison of virus reactivation, DNA base damage, and cell cycle effects in autologous human melanoma cells resistant to methylating agents. Cancer Res. 1984 Jan;44(1):55–58. [PubMed] [Google Scholar]
- Hora J. F., Eastman A., Bresnick E. O6-methylguanine methyltransferase in rat liver. Biochemistry. 1983 Aug 2;22(16):3759–3763. doi: 10.1021/bi00285a007. [DOI] [PubMed] [Google Scholar]
- Jun G. J., Ro J. Y., Kim M. H., Park G. H., Paik W. K., Magee P. N., Kim S. Studies on the distribution of O6-methylguanine-DNA methyltransferase in rat. Biochem Pharmacol. 1986 Feb 1;35(3):377–384. doi: 10.1016/0006-2952(86)90208-x. [DOI] [PubMed] [Google Scholar]
- Kyrtopoulos S. A., Ampatzi P., Davaris P., Haritopoulos N., Golematis B. Studies in gastric carcinogenesis. IV. O6-methylguanine and its repair in normal and atrophic biopsy specimens of human gastric mucosa. Correlation of O6-alkylguanine-DNA alkyltransferase activities in gastric mucosa and circulating lymphocytes. Carcinogenesis. 1990 Mar;11(3):431–436. doi: 10.1093/carcin/11.3.431. [DOI] [PubMed] [Google Scholar]
- Lee S. M., Thatcher N., Margison G. P. O6-alkylguanine-DNA alkyltransferase depletion and regeneration in human peripheral lymphocytes following dacarbazine and fotemustine. Cancer Res. 1991 Jan 15;51(2):619–623. [PubMed] [Google Scholar]
- Maynard K., Parsons P. G., Cerny T., Margison G. P. Relationships among cell survival, O6-alkylguanine-DNA alkyltransferase activity, and reactivation of methylated adenovirus 5 and herpes simplex virus type 1 in human melanoma cell lines. Cancer Res. 1989 Sep 1;49(17):4813–4817. [PubMed] [Google Scholar]
- Myrnes B., Norstrand K., Giercksky K. E., Sjunneskog C., Krokan H. A simplified assay for O6-methylguanine-DNA methyltransferase activity and its application to human neoplastic and non-neoplastic tissues. Carcinogenesis. 1984 Aug;5(8):1061–1064. doi: 10.1093/carcin/5.8.1061. [DOI] [PubMed] [Google Scholar]
- O'Connor P. J., Fida S., Fan C. Y., Bromley M., Saffhill R. Phenobarbital: a non-genotoxic agent which induces the repair of O6-methylguanine from hepatic DNA. Carcinogenesis. 1988 Nov;9(11):2033–2038. doi: 10.1093/carcin/9.11.2033. [DOI] [PubMed] [Google Scholar]
- Parsons P. G., Smellie S. G., Morrison L. E., Hayward I. P. Properties of human melanoma cells resistant to 5-(3',3'-dimethyl-1-triazeno)imidazole-4-carboxamide and other methylating agents. Cancer Res. 1982 Apr;42(4):1454–1461. [PubMed] [Google Scholar]
- Pegg A. E. Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res. 1990 Oct 1;50(19):6119–6129. [PubMed] [Google Scholar]
- Pegg A. E., Wiest L., Foote R. S., Mitra S., Perry W. Purification and properties of O6-methylguanine-DNA transmethylase from rat liver. J Biol Chem. 1983 Feb 25;258(4):2327–2333. [PubMed] [Google Scholar]
- Potter P. M., Rafferty J. A., Cawkwell L., Wilkinson M. C., Cooper D. P., O'Connor P. J., Margison G. P. Isolation and cDNA cloning of a rat O6-alkylguanine-DNA-alkyltransferase gene, molecular analysis of expression in rat liver. Carcinogenesis. 1991 Apr;12(4):727–733. doi: 10.1093/carcin/12.4.727. [DOI] [PubMed] [Google Scholar]
- Przepiorka D., Myerson D. A single-step silver enhancement method permitting rapid diagnosis of cytomegalovirus infection in formalin-fixed, paraffin-embedded tissue sections by in situ hybridization and immunoperoxidase detection. J Histochem Cytochem. 1986 Dec;34(12):1731–1734. doi: 10.1177/34.12.3023477. [DOI] [PubMed] [Google Scholar]
- Rafferty J. A., Elder R. H., Watson A. J., Cawkwell L., Potter P. M., Margison G. P. Isolation and partial characterisation of a Chinese hamster O6-alkylguanine-DNA alkyltransferase cDNA. Nucleic Acids Res. 1992 Apr 25;20(8):1891–1895. doi: 10.1093/nar/20.8.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard A., Verly W. G. A chromatin factor in rat liver which destroys O6-ethylguanine in DNA. FEBS Lett. 1980 May 19;114(1):98–102. doi: 10.1016/0014-5793(80)80868-4. [DOI] [PubMed] [Google Scholar]
- Sagher D., Karrison T., Schwartz J. L., Larson R., Meier P., Strauss B. Low O6-alkylguanine DNA alkyltransferase activity in the peripheral blood lymphocytes of patients with therapy-related acute nonlymphocytic leukemia. Cancer Res. 1988 Jun 1;48(11):3084–3089. [PubMed] [Google Scholar]
- Santibanez-Koref M., Elder R. H., Fan C. Y., Cawkwell L., McKie J. H., Douglas K. T., Margison G. P., Rafferty J. A. Isolation and partial characterization of murine O6-alkylguanine-DNA-alkyltransferase: comparative sequence and structural properties. Mol Carcinog. 1992;5(2):161–169. doi: 10.1002/mc.2940050212. [DOI] [PubMed] [Google Scholar]
- Wiestler O., Kleihues P., Pegg A. E. O6-alkylguanine-DNA alkyltransferase activity in human brain and brain tumors. Carcinogenesis. 1984 Jan;5(1):121–124. doi: 10.1093/carcin/5.1.121. [DOI] [PubMed] [Google Scholar]
- Wilkinson M. C., Potter P. M., Cawkwell L., Georgiadis P., Patel D., Swann P. F., Margison G. P. Purification of the E. coli ogt gene product to homogeneity and its rate of action on O6-methylguanine, O6-ethylguanine and O4-methylthymine in dodecadeoxyribonucleotides. Nucleic Acids Res. 1989 Nov 11;17(21):8475–8484. doi: 10.1093/nar/17.21.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]