Abstract
The detrimental effects of elevated intensities of mid-UV radiation (UVB), a result of stratospheric ozone depletion during the austral spring, on the primary producers of the Antarctic marine ecosystem have been well documented. Here we report that natural populations of Antarctic zooplankton also sustain significant DNA damage [measured as cyclobutane pyrimidine dimers (CPDs)] during periods of increased UVB flux. This is the first direct evidence that increased solar UVB may result in damage to marine organisms other than primary producers in Antarctica. The extent of DNA damage in pelagic icefish eggs correlated with daily incident UVB irradiance, reflecting the difference between acquisition and repair of CPDs. Patterns of DNA damage in fish larvae did not correlate with daily UVB flux, possibly due to different depth distributions and/or different capacities for DNA repair. Clearance of CPDs by Antarctic fish and krill was mediated primarily by the photoenzymatic repair system. Although repair rates were large for all species evaluated, they were apparently inadequate to prevent the transient accumulation of substantial CPD burdens. The capacity for DNA repair in Antarctic organisms was highest in those species whose early life history stages occupy the water column during periods of ozone depletion (austral spring) and lowest in fish species whose eggs and larvae are abundant during winter. Although the potential reduction in fitness of Antarctic zooplankton resulting from DNA damage is unknown, we suggest that increased solar UV may reduce recruitment and adversely affect trophic transfer of productivity by affecting heterotrophic species as well as primary producers.
Keywords: ozone depletion, DNA damage and repair, photolyase, marine ecosystems
The concentration of stratospheric ozone has decreased significantly during the past two decades, the result of catalytic destruction of ozone mediated by the photodegradation products of anthropogenic chlorofluorocarbons (1, 2). Ozone depletion has been most dramatic over Antarctica, where ozone levels typically decline >50% during the austral spring “ozone hole” (3–5). Elsewhere, ozone concentrations have fallen gradually at temperate latitudes (6, 7), and further depletion over a broader geographical range is anticipated during the next 25–100 years (7, 8). Atmospheric ozone strongly and selectively absorbs solar UVB (290–320 nm), thus reducing the intensity of the most biologically damaging solar wavelengths that penetrate the atmosphere (9), and decreased stratospheric ozone has been linked directly to increased UVB flux at the earth’s surface (10). UVB penetrates to ecologically significant depths (20–30 m) in the ocean at intensities that cause measurable biological damage (11–16). Therefore, the fitness of marine organisms in coastal regions and the upper photic zone of open oceans may be affected deleteriously by the projected long-term increase in UVB flux (16–20).
Although UVB damages most biological macromolecules, including lipids, proteins, and nucleic acids (21, 22), the principal cause of UV-induced mutation, degeneration, and/or death in animal cells is modification of DNA (23). The most significant DNA lesions generated by UVB are cyclobutane pyrimidine dimers (CPDs), which constitute ≈70–90% of the aberrant DNA photoproducts (23). CPDs increase linearly with UVB exposure (23, 24), but the dose–response relationship varies significantly between different species and taxa (25–27). Nevertheless, the CPD burden resulting from sublethal doses of UVB may inhibit embryonic and larval development and ultimately decrease survival, by slowing transcription and mitosis and by imposing energetic costs associated with DNA repair (28).
The impact of elevated UVB on marine ecosystems has been documented most extensively for the primary producers of polar latitudes (refs. 13 and 29; reviewed in ref. 30). Primary productivity in the Southern Ocean declines by as much as 15% in areas affected by the ozone hole (13). Survival of and photosynthesis by Antarctic diatoms are reduced by UVB, although the susceptibility of these single-celled algae to UVB-induced DNA damage varies significantly, depending upon cell surface:volume ratios, pigmentation, and DNA repair rates (25). UVB has also been shown to inhibit nitrogen uptake by phytoplankton from the North Atlantic (31). Although previous investigators have strongly suggested that elevated UVB flux may perturb marine ecosystems as a whole (32, 33), its effects on marine organisms other than primary producers have not yet been systematically investigated. Bothwell et al. (34), after demonstrating that herbivorous grazers were the keystone species affected by UVB in shallow temperate streams, concluded that “predictions of the response of entire ecosystems to elevated UVB cannot be made on single trophic-level assessments.”
Most organisms possess defense mechanisms that act either to prevent UVB-induced DNA damage (via behavioral, physical, and/or chemical strategies) or to repair it after it has occurred. The two primary repair systems are photoenzymatic repair (PER; “light repair”) and nucleotide excision repair (NER; “dark repair”) (25). PER repairs primarily CPDs via the enzyme photolyase, which uses near-UV light (320–400 nm) as its source of energy. NER, by contrast, involves multiple proteins (e.g., UVR-A, -B, and -C in Escherichia coli), does not require light for catalysis and repairs preferentially the 6–4 pyrimidine–pyrimidinone photoproduct and the Dewar pyrimidinone (35).
To assess the potential impact of ozone depletion and increased UVB intensities on populations of marine heterotrophs in Antarctica, we analyzed field-collected zooplankton for UVB-induced DNA damage (CPDs) during the ozone hole (October–November) of 1994. Because DNA damage and repair occur contemporaneously in the field, we also evaluated the ability of three Antarctic heterotrophs [two teleosts, Notothenia coriiceps (Nototheniidae; rockcods) and Chaenocephalus aceratus (Channichthyidae; icefishes) and one species of krill, Euphausia superba] to repair UV-induced DNA damage at ambient temperatures (−1 to +1°C). Finally, to evaluate the relative efficiency of DNA repair in these cold-adapted Antarctic species, we measured DNA repair rates of a temperate, eurythermal teleost (Fundulus heteroclitus) at three acclimation temperatures. Our results indicate that Antarctic zooplankters accumulate significant CPD levels during periods of increased UVB flux, that repair of this damage is mediated largely by the photoenzymatic repair system, and that DNA repair capacity is highest for species whose eggs and larvae occupy the water column during the austral spring. Although the reduction in zooplankton fitness resulting from this DNA damage is unknown, we suggest that increased solar UVB flux may reduce recruitment and trophic transfer of productivity by affecting both the heterotrophic species and the primary producers of the Antarctic marine ecosystem.
MATERIALS AND METHODS
Zooplankton Collections.
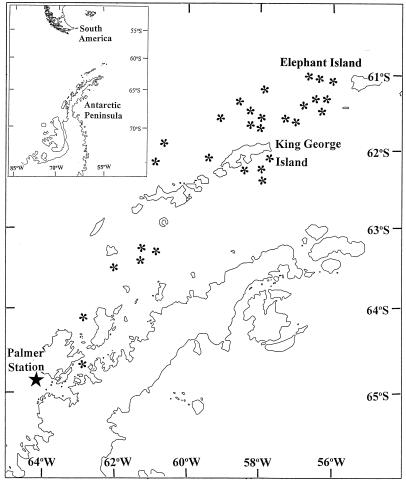
Macrozooplankton was collected from Antarctic surface waters (0–35 m deep) of the Palmer Archipelago (see Fig. 1 for sampling locations) during the austral spring of 1994 (R/V Polar Duke cruise 94-10, October 13–November 16). Specimens included fish larvae, fish eggs (unhatched, late-somitic developmental stages), chaetognaths, and annelids. Each sampling event was conducted for 1–2 h, during daylight hours (0600–1800), with a 2 × 3 m rectangular midwater trawl equipped with a 3-mm nylon delta mesh net. Each sampling event fished at a constant depth, estimated by wire angle and length of wire deployed. Organisms from net samples were identified (36), sorted by taxa, frozen in liquid nitrogen, and stored in the dark at −70°C until analysis for CPDs.
Figure 1.
Antarctic zooplankton sampling locations in the Palmer Archipelago during October 1994–November 1994. Asterisks indicate stations where zooplankton was collected.
Ethanol-preserved Notothenia larseni larvae were obtained from Richard Radtke (University of Hawaii) to represent similar macrozooplankton collected at similar depths (0–45 m) during “normal” ozone conditions (December 1988–January 1989). These samples, derived from the Meteor Cruise ME11/4 (37), were stored in UV-absorbing glass vials before use.
UVB Measurements.
Cumulative daily solar UVB (290–320 nm) flux, weighted to a biological action spectrum determined for larval fish (38), was measured by the National Science Foundation Monitoring Station at nearby Palmer Station (data provided by Biospherical Instruments, San Diego). Hunter’s weighted UVB flux (μW/cm2), summed for each day (0000–2359 h) of cruise 94-10, was used as an estimate of biologically damaging surface UVB flux.
Quantitation of Photoproducts.
Genomic DNA was purified from individual fish larvae, chaetognaths, or annelids and from samples (n = 78) of pooled icefish eggs (2–4 eggs per sample gave 15–50 μg of DNA per sample), by the method outlined in ref. 39. Ethanol-preserved fish larvae were rehydrated in TE buffer (10 mM Tris·HCl/1 mM EDTA, pH 8) for 24 h at 4°C before extraction of DNA. Duplicate aliquots of DNA (5 μg) from each sample were analyzed for CPD content by the competitive radioimmunoassay of Mitchell et al. (40). Briefly, unlabeled, heat-denatured specimen DNA, 32P-labeled, UVB-irradiated poly(dA):poly(dT) competitive antigen (10 pg; 5 × 108 cpm/μg by random priming; 800 kJ/m2 at 320 nm), and carrier salmon sperm DNA (5 μg/ml) were mixed, and rabbit antiserum (1:1000 dilution) specific for UV-damaged DNA was incubated with the sample for 1–2 h at 37°C. Immune complexes were collected by precipitation overnight at 4°C with goat anti-rabbit IgG and carrier IgG followed by centrifugation. After washing, pellets were dissolved in NCS II solubilizer (Amersham), and radioactivity was quantified by liquid scintillation counting in Scintiverse (Fisher). Specimen DNA damage (CPDs per Mb) was evaluated by use of a standard curve generated with unlabeled DNA containing known quantities of CPDs.
DNA Repair Measurements.
To determine the relative importance of PER and NER repair systems in Antarctic marine heterotrophs, we measured light and dark DNA repair rates of juvenile specimens of two abundant fish species (the rockcod N. coriiceps and the icefish C. aceratus) and of adult specimens of krill (E. superba). The fishes, whose embryonic and larval stages differ in seasonal exposure to solar illumination (see Results), were collected during R/V Polar Duke cruises 94-10, 95-2, and 95-3 (October 1994–November 1994, March 1995, and May 1995, respectively). Krill were collected with a plankton net from surface waters near Palmer Station in February 1995. As a benchmark for comparison of DNA repair efficiency in these cold-living poikilotherms, we also evaluated repair rates of the eurythermal killifish, F. heteroclitus, at three acclimation temperatures (6, 10, and 25°C). This species [collected by minnow trap at Woods Hole, MA (June, 1995)] is an abundant component of most estuarine habitats on the East coast of the United States (41), with a latitudinal distribution from Newfoundland to south Florida (42). Its primary habitats are shallow saltmarsh creeks, in which it may encounter seasonal temperature ranges of −2°C in winter to +35°C in summer. Fundulus eggs typically develop out of the water for periods up to 4 weeks during the spring and summer, which makes them particularly vulnerable to UVB.
These organisms were acclimated to experimental temperatures for a minimum of 14 days and held in the dark for 24 h before exposure to 500-2500 J/m2 UVB [generated by 5 × 8 W UVB lamps (Spectronic, Westbury NY), peak emission 312 nm, in a Fisher Biotech UV Crosslinker]. Immediately following irradiation, specimens used in the PER studies were exposed to continuous photoreactivating light (one General Electric 15-W Cool White fluorescent bulb at a distance of 25 cm) and then sacrificed at intervals for evaluation of CPD burden. DNAs from fin tissues of the Antarctic fishes, from fins and muscle-free skin of the killifish, and from whole krill were purified and analyzed for CPDs by radioimmunoassay as described above. PER rates were calculated by fitting the time course of CPD disappearance to the function CPD = e−R(t+1), where CPD is the percentage change in dimer concentration, R is the relative PER rate, and t is the post-UV irradiation time (in h). R was calculated by determining the least-squares slope of the logarithm-transformed relationship between CPD and time. Dark repair (NER) was evaluated with an identical protocol, but specimens were held in the dark for the duration of post-irradiation sampling.
RESULTS
DNA Damage in Natural Populations of Antarctic Zooplankton.
Eggs (unhatched, late-somitic stages) and larvae of the hemoglobinless icefish C. aceratus (Channichthyidae) predominated in the zooplankton collections, but chaetognaths and transparent planktonic polychaetes were also represented (Table 1). During the period of sample collection, cumulative daily spectral irradiance values (290–320 nm) at nearby Palmer Station (Fig. 1) peaked at 1308 μW/cm2 (Hunter’s UVB = 7.42) on October 31 but varied considerably [Hunter’s UVB = 4.08 ± 1.85 (mean ± SD)]. Table 1 shows that a large proportion (>50%) of specimens in each taxon contained DNA damage. Ichthyofauna (eggs and larvae of C. aceratus) contained the highest levels of damage, both in CPD burden (average of 31–35 CPDs per Mb) and in the proportion of damaged specimens (>67%). In contrast, the N. larseni larvae, which are similar in size and coloration to the C. aceratus specimens but were collected during a period of lower UVB flux [daily fluxes in the range 413–666 μW/cm2 (Hunter’s UVB = 2.12–3.27), December 12–28, 1988] (T. Mestechkina, personal communication), had no detectable DNA damage. The sample sizes for chaetognaths and polychaetes were small, but half or more of these specimens also contained DNA damage, albeit at lower levels than those of the ichthyofauna. To our knowledge, this is the first demonstration that ozone depletion may be causing measurable DNA damage in natural populations of Antarctic heterotrophs.
Table 1.
DNA damage (CPDs per Mb of DNA) measured in natural populations of Antarctic marine macrozooplankton during depleted (October 1994–November 1994) and normal (December 1988) ozone conditions
| Collection date | Phylum | Family | n* | CPDs per Mb, mean (SEM) | Percent with measurable damage |
|---|---|---|---|---|---|
| Oct./Nov. 1994† | Annelida | Alciopidae | 2 | 4 (4) | 50 |
| Chaetognatha | ? | 6 | 10.6 (5.8) | 66.6 | |
| Chordata | Channichthyidae-eggs | 78 | 31.0 (9.9) | 67.5 | |
| larvae-anterior | 35 | 35.1 (8.8) | 80 | ||
| larvae-posterior | 35 | 31.0 (8.7) | 68.6 | ||
| Dec. 1988‡ | Chordata | Nototheniidae (larvae) | 17 | 0 (0) | 0 |
Genomic DNA samples were prepared from 2–4 eggs or from one larvae; n represents the number of DNA samples analyzed by radioimmunoassay.
See Fig. 1 for collection areas.
Fish larvae were provided by Richard Radtke (University of Hawaii).
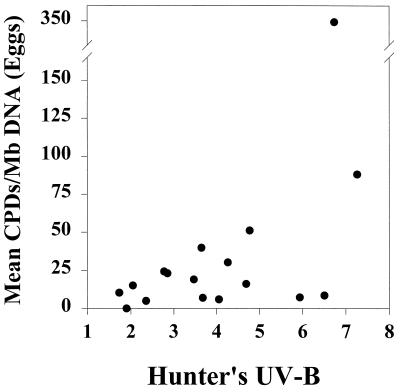
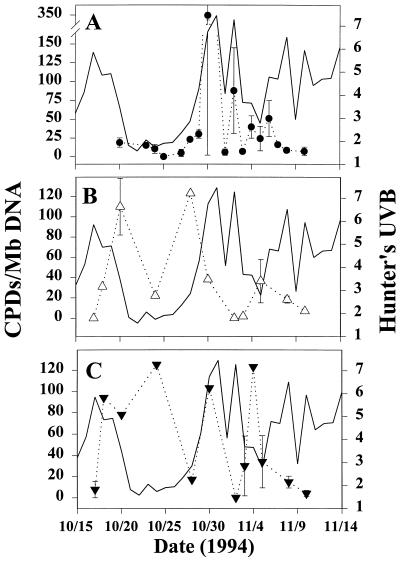
DNA damage of C. aceratus eggs (daily means) correlated significantly with cumulative daily UVB flux (P < 0.05, Pearson’s correlation) (Fig. 2). Furthermore, mean CPD burden in C. aceratus eggs paralleled closely the biologically weighted surface UVB irradiance levels (Fig. 3A), which suggests strongly that DNA damage in these epipelagic zooplankton is largely attributable to solar UVB. CPD levels in C. aceratus larvae (heads and bodies analyzed separately), by contrast, tracked less closely with UVB intensity (Fig. 3 B and C), perhaps due to differences in UV absorptivity and/or DNA repair capacity relative to eggs. No significant correlation was found between CPD content and depth of collection for any of the taxa examined (Pearson’s, α = 0.05).
Figure 2.
Mean daily CPD content of icefish eggs as a function of biologically weighted daily UVB flux. DNA damage (CPDs per Mb of DNA) is plotted vs. Hunter’s weighted UVB for each day during which samples were collected. CPD content correlated significantly with cumulative daily UVB flux (P < 0.05, Pearson’s correlation).
Figure 3.
Temporal covariation of DNA damage in Antarctic ichthyoplankton with ambient UVB flux during austral spring of 1994. CPD levels (CPD per Mb of DNA; mean ± SEM) for icefish (C. aceratus) eggs (A, •) and for larval icefish heads (B, ▵) and bodies (C, ▾) are plotted vs. date of specimen capture. Biologically weighted cumulative daily solar UVB (Hunter’s UVB), measured at Palmer Station, is indicated by the solid line in each panel.
DNA Repair Rates of Antarctic Organisms.
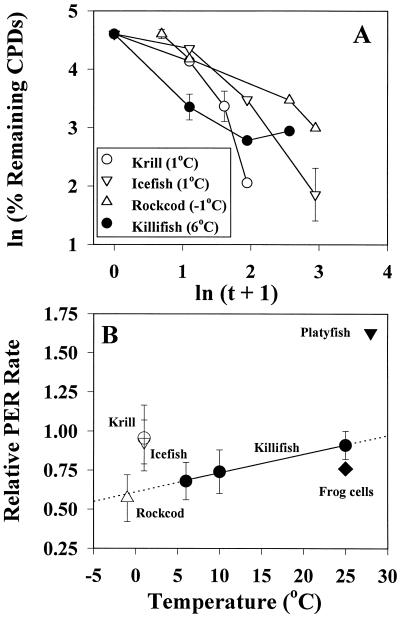
In both species of Antarctic fish and in krill, CPDs were repaired rapidly by PER at physiological temperatures (Table 2, Fig. 4 A and B). For the eurythermal killifish, the rate of PER increased as a linear function of temperature (Fig. 4B), almost spanning from 6 to 25°C the range of rates observed for the Antarctic organisms at −1 to +1°C. Clearance of CPDs by NER, by contrast, was considerably slower (typically < 15% reduction in CPDs after 24 h) for both the cold-adapted and temperate organisms (Table 2). Thus, PER appears to be the major DNA repair system available to these marine species, but the ontogeny of PER and NER remains to be examined.
Table 2.
PER and NER rates of Antarctic fishes, krill, and the temperate killifish
| Organism* | T, °C | PER rate† | n‡ | r2 | t50%,§ h | t80%,§ h | NER rate† | n‡ | r2 |
|---|---|---|---|---|---|---|---|---|---|
| Rockcod | −1 | 0.57 | 3 | 0.97 | 2.37 | 15.8 | 0.003 | 2 | 0.42 |
| Icefish | 1 | 0.93 | 2 | 0.88 | 1.11 | 4.6 | 0.006 | 2 | 0.64 |
| Krill | 1 | 0.96 | 2 | 0.53 | 1.06 | 4.4 | 0.008 | 2 | 0.51 |
| Killifish | 6 | 0.68 | 3 | 0.85 | 1.77 | 9.7 | 0.012 | 2 | 0.48 |
| 10 | 0.74 | 3 | 0.80 | 1.55 | 7.8 | 0.024 | 2 | 0.54 | |
| 25 | 0.91 | 3 | 0.69 | 1.14 | 4.9 | 0.022 | 2 | 0.56 |
Rockcod, N. coriiceps; icefish, C. aceratus; krill, E. superba; and killifish, F. heteroclitus.
PER and NER rates were calculated by fitting, by the method of least squares, the temporal dependence of CPD clearance under photoreactivating (Fig. 4A) and dark conditions, respectively, to the equation: ln(% remaining CPDs) = −R·ln(t + 1) + ln(100%), where t is hours after irradiation and R is the PER or NER rate.
n indicates the number of independent repair rate measurements; representative time courses of CPD clearance by PER are shown in Fig. 4A.
t50% and t80% are the times required for repair of 50% and 80% of CPDs, respectively, under photoreactivating conditions.
Figure 4.
DNA repair by PER in Antarctic fish, temperate fish, and krill. (A) Temporal dependence of CPD clearance for fin tissues of Antarctic fish (N. coriiceps, ▵; C. aceratus, ▿), for fin and skin tissues of the temperate killifish (F. heteroclitus, •), and for whole krill (E. superba, ○). Post-irradiation time, in h, is represented by t. Experimental temperatures are indicated, and representative error bars are given for each time course. (B) Temperature dependence of PER rates (mean ± SD) for Antarctic fish, the killifish, and krill (symbols as in A). PER rates for the eurythermal killifish were determined at three acclimation temperatures; the solid line is the best-fitting linear regression and its dotted extensions extrapolate the trend in the data to lower and higher temperatures. Also shown are data for the tropical teleost Xiphophorous (platyfish; ref. 43) and for cultured ICR 2A frog cells (40).
Within the Antarctic taxa, rates of DNA repair appear to correlate with the life histories of their embryonic stages. Particularly noteworthy are the high DNA repair capacities of icefish and krill, whose eggs and larvae remain in the water column throughout the austral spring and summer (44); at +1°C their PER rates are comparable to those observed for the killifish at 25°C (Fig. 4B). By contrast, PER rates for the rockcod, whose eggs and larvae develop in Antarctic surface waters during seasons of low solar illumination (austral fall and winter; ref. 44), are ≈50% smaller, matching the value extrapolated for the temperate fish (Fig. 4B, dotted line). These differences are exemplified by the time required for 80% repair of initial CPD loads, which ranged from 4 to 5 h for the icefish and krill (+1°C) to >15 h for the rockcod (−1°C; Table 2). If PER activities in icefish eggs and larvae are comparable to that measured in juveniles, then the daily tracking of DNA damage with UVB flux (Fig. 3) is readily interpreted as the difference between temporally integrated damage and first-order CPD repair.
DISCUSSION
The detrimental effects of increased solar UVB, caused by stratospheric ozone depletion, on single-celled phytoplankton have been known for several years (13, 29, 30). We show here that natural levels of solar UVB during ozone depletion may also cause measurable damage to multicellular organisms occupying higher trophic levels of the Antarctic marine ecosystem. The level of UVB-induced DNA damage measured in ichthyoplankton is greater than the lethal limit previously determined for Antarctic diatoms (>15 CPDs per Mb) and is comparable to the lethal limit of DNA damage for cultured goldfish cells (20–100 CPDs per Mb) (25, 45). Despite substantial temporal changes in solar intensity, cloud cover, water column turbidity, and vertical mixing of the zooplankters during the sampling period, more than half of all specimens contained measurable DNA damage at levels that are probably physiologically relevant.
DNA damage was greater in the fish specimens than in the other taxa examined. In particular, the CPD content of icefish eggs closely followed daily UVB flux, which suggests that damage accrued during each 24-h period was repaired in less than 1 day (validating laboratory-derived DNA repair rates; see below). Icefish eggs, which are abundant, buoyant, and consistent in size, shape, and transparency, may therefore serve as useful biological indicators of the DNA-damaging effects of UVB in those zooplankters confined to Antarctic surface waters.
Icefish larvae, by contrast, showed patterns of DNA damage that correlated less well with daily UVB flux. Factors that may explain the distinct damage patterns observed for eggs and larvae include differences in: (i) UV absorptivity due to surface:volume ratio, pigmentation, content of UV-absorbing compounds, and/or mobility in the water column and (ii) DNA repair capacity. At present, the data required to discriminate between these possibilities is only suggestive. Icefish larvae contain moderate to high levels of UV-absorbing compounds (46), but the status of such materials in icefish eggs is unknown. DNA repair occurs at greater rates in young, undifferentiated cells (47), which suggests that DNA damage tracks ambient UVB more closely in icefish eggs than in larvae because eggs may be able to clear CPDs more rapidly. However, the expression and function of DNA repair systems during icefish development remains to be determined.
Assessment of the potential effect of elevated solar UVB on marine organisms requires consideration of light attenuation, mixing depth and sea state. Light intensity decreases exponentially with depth of the water column, yet biologically damaging levels of UV can penetrate to depths >30 m (11–16). Variability in sea state, turbidity, and vertical mixing/migration rates can influence substantially the actual damage accruing to marine organisms (11, 13, 14). For example, Jeffrey et al. (16) showed recently that sea state and vertical mixing rate are inversely proportional to the level of solar UV-induced DNA damage measured in marine bacteria in the Gulf of Mexico. Because we cannot reconstruct the precapture depth history of individual zooplankters in our field collections (depth of capture need not correspond to temporally weighted average depth of UVB exposure), it is not surprising that we found no relationship between collection depth and CPD load. Furthermore, diel and ontogenetic patterns of depth distribution are required to fully evaluate the impact of solar UVB on any marine taxon. We suggest, therefore, that buoyant, relatively nonmobile fish eggs may serve as effective passive in situ dosimeters, but accurate assessment of the effect of solar UVB on highly mobile taxa will require knowledge of vertical mixing rates and diel migratory behavior.
Antarctic fish and krill appear to rely primarily on PER, and therefore the single-enzyme system photolyase, to remove UVB-induced CPDs, as do several other taxa (25, 26, 40, 43). Each of the Antarctic species examined in this study demonstrated high repair capacities at the low ambient temperatures that characterize the Southern Ocean (−2 to +2°C), but rate differences possibly associated with the seasonality of embryogenesis were detected. Thus, the PER rates of the icefish C. aceratus and the krill E. superba, whose eggs and larvae are present in surface waters when solar illumination is high and ozone is depleted (austral spring and summer; ref. 48), are approximately twice as large as that of the rockcod N. coriiceps, whose eggs and larvae, spawning and developing during austral fall and winter, are exposed to much lower solar intensities. Although the data are preliminary, we hypothesize that Antarctic zooplankton whose early life history stages develop in the water column during periods of sustained, intense solar illumination, and hence are exposed to higher intensities of solar UV even in the absence of ozone depletion, may have increased capacities for DNA repair. Further evaluation of the PER systems of other Antarctic taxa would be required to test this proposal.
Comprehensive sampling of Antarctic zooplankton and field manipulation of their exposure to UVB, during both ozone-depleted and normal conditions, and determination of the effect of CPDs on the fitness of these organisms are required before the large-scale impact of ozone depletion on Antarctic marine heterotrophs can be fully evaluated. Our data suggest, however, that the effect on organisms occupying trophic levels other than primary producers may be substantial. Larval and adult fish, krill, copepods, and gelatinous zooplankton are all important components of the truncated trophic structure of the Southern Ocean (44, 48–51). They generally have transparent eggs, larvae, and/or adult stages that are pelagic, planktonic, and often remain in surface waters for 6–12 months (44, 49–54). Thus, these species are potentially vulnerable to DNA damage from the elevated UVB fluxes that now occur during the austral spring. The levels of DNA damage that we measured in natural populations of Antarctic zooplankton suggest that tissue damage accruing from UVB exposure may be of sufficient magnitude to decrease the fitness of developing eggs and larvae, thereby reducing recruitment. Thus, elevated solar UVB flux during the austral spring may have a substantial impact on populations of both primary producers and heterotrophs of the Antarctic marine ecosystem.
Acknowledgments
We gratefully acknowledge the staff of the Office of Polar Programs of the National Science Foundation, the personnel of Antarctic Support Associates, and the captain and crew of R/V Polar Duke for their logistic support of the field research. We thank Ralph Pledger (University of Texas) for technical assistance with the radioimmunoassay, Richard Radtke for contributing the N. larseni larvae, and Karen Haberman (University of California) for providing krill. Tim Lucas and Tanya Mestechkina of Biospherical Instruments, San Diego, CA, provided the UVB data. This work was supported by National Science Foundation Grants OPP-9408713 (H.W.D.) and OPP-9420712 (H.W.D.) and by a Marine Biological Laboratory/Frederik B. Bang Research Fellowship (K.D.M.) at the Marine Biological Laboratory, Woods Hole, MA.
Footnotes
Abbreviations: CPD, cyclobutane pyrimidine dimer; PER, photoenzymatic repair; NER, nucleotide excision repair.
References
- 1.Anderson J G, Toohey D W, Brune W H. Science. 1991;251:39–46. doi: 10.1126/science.251.4989.39. [DOI] [PubMed] [Google Scholar]
- 2.Schoeberl M R, Hartmann D L. Science. 1991;251:46–52. doi: 10.1126/science.251.4989.46. [DOI] [PubMed] [Google Scholar]
- 3.Frederick J E, Snell H E. Science. 1988;241:438–440. doi: 10.1126/science.241.4864.438. [DOI] [PubMed] [Google Scholar]
- 4.Brasseur G. Environment. 1987;29:6–11. [Google Scholar]
- 5.Solomon S. Nature (London) 1990;347:347–354. [Google Scholar]
- 6.Madronich S, De Gruijl F R. Photochem Photobiol. 1994;59:541–546. doi: 10.1111/j.1751-1097.1994.tb02980.x. [DOI] [PubMed] [Google Scholar]
- 7.Jones A E, Shanklin J D. Nature (London) 1995;376:409–411. [Google Scholar]
- 8.Crawford M. Science. 1987;237:1557. doi: 10.1126/science.237.4822.1557. [DOI] [PubMed] [Google Scholar]
- 9.Molina L T, Molina M J. J Geophys Res Atmos. 1986;91:14501–14508. [Google Scholar]
- 10.Lubin D, Frederick J E, Booth C R, Lucas T, Neuschuler D. Geophys Res Lett. 1989;16:783–785. [Google Scholar]
- 11.Smith R C, Baker K S. Photochem Photobiol. 1979;29:311–323. [Google Scholar]
- 12.Calkins J, Thordardottir T. Nature (London) 1980;283:563–566. [Google Scholar]
- 13.Smith R C, Prezelin B B, Baker K S, Bidigare R R, Boucher N P, Coley T, Karentz D, MacIntyre S, Matlick H A, Menzies D, Ondrusek M, Wan Z, Waters K J. Science. 1992;255:952–959. doi: 10.1126/science.1546292. [DOI] [PubMed] [Google Scholar]
- 14.Karentz D, Cleaver J E, Mitchell D L. Nature (London) 1991;350:28. [Google Scholar]
- 15.Calkins J. In: The Role of Ultraviolet Radiation in Marine Ecosystems. Calkins J, editor. New York: Plenum; 1982. pp. 169–179. [Google Scholar]
- 16.Jeffrey W H, Aas P, Maille Lyons M, Coffin R B, Pledger R J, Mitchell D L. Photochem Photobiol. 1996;64:419–427. [Google Scholar]
- 17.Worrest R C, Brooker D L, Van Dyke H. Limnol Oceanogr. 1980;25:360–364. [Google Scholar]
- 18.Damkaer D M, Dey D B, Heron G A. Oecologia. 1981;48:178–182. doi: 10.1007/BF00347960. [DOI] [PubMed] [Google Scholar]
- 19.Worrest R C. In: The Role of Solar Ultraviolet Radiation in Marine Ecosystems. Calkins J, editor. New York: Plenum; 1982. pp. 429–457. [Google Scholar]
- 20.Cullen J J, Lesser M P. Mar Biol (Berlin) 1991;111:183–190. [Google Scholar]
- 21.Sauerbier W. Adv Radiat Biol. 1976;6:49–106. [Google Scholar]
- 22.Kantor G J, Hull D R. Biophys J. 1979;27:359–370. doi: 10.1016/S0006-3495(79)85223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrier W L, Snyder R D, Regan J D. In: The Science of Photomedicine. Regan J D, Parrish J A, editors. New York: Plenum; 1982. pp. 91–112. [Google Scholar]
- 24.Setlow R B. Proc Natl Acad Sci USA. 1974;71:3363–3366. doi: 10.1073/pnas.71.9.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell D L, Karentz D. In: Environmental UV Photobiology. Young A R, Bjorn L O, Moan J, Nultsch W, editors. New York: Plenum; 1993. pp. 345–377. [Google Scholar]
- 26.Blaustein A R, Hoffman P D, Hokit D G, Kiesecker J M, Walls S C, Hays J B. Proc Natl Acad Sci USA. 1994;91:1791–1795. doi: 10.1073/pnas.91.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlini D B, Regan J D. J Exp Mar Biol Ecol. 1995;219:219–232. [Google Scholar]
- 28.Blakefield M I, Harris D O. Photochem Photobiol. 1994;59:204–208. doi: 10.1111/j.1751-1097.1994.tb05023.x. [DOI] [PubMed] [Google Scholar]
- 29.Ryan K G. J Photochem Photobiol B. 1992;13:235–240. [Google Scholar]
- 30.Weiler, C. S. & Penhale, P. A., eds. (1994) Ultraviolet Radiation in Antarctica: Measurements and Biological Effects, Antarctic Research Series (Am. Geophys. Union, Washington, DC), Vol. 62.
- 31.Dohler G. Mar Biol (Berlin) 1992;112:485–490. [Google Scholar]
- 32.Karentz D. Antarct Sci. 1991;3:3–11. [Google Scholar]
- 33.Roberts L. Science. 1989;244:288–289. doi: 10.1126/science.244.4902.288. [DOI] [PubMed] [Google Scholar]
- 34.Bothwell M L, Sherbot D M J, Pollock C M. Science. 1994;265:97–100. doi: 10.1126/science.265.5168.97. [DOI] [PubMed] [Google Scholar]
- 35.Sancar A. Science. 1994;266:1954–1957. doi: 10.1126/science.7801120. [DOI] [PubMed] [Google Scholar]
- 36.Kellerman, A. (1989) in Catalogue of Early Life History Stages of Antarctic Notothenioid Fishes, BIOMASS Science Series No. 10, ed. Kellerman, A. (Alfred-Wegener-Institut, Bremerhaven, Germany), pp. 45–136.
- 37.Siegel V. Arch Fischereiwiss. 1992;41:101–130. [Google Scholar]
- 38.Hunter J R, Taylor J H, Moser H G. Photochem Photobiol. 1979;29:325–338. doi: 10.1111/j.1751-1097.1979.tb07055.x. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T, editors. Molecular Cloning. Vol. 2. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. pp. 9.16–9.19. [Google Scholar]
- 40.Mitchell D L, Clarkson J M, Chao C C-K, Rosenstein B S. Photochem Photobiol. 1986;43:595–597. doi: 10.1111/j.1751-1097.1986.tb09539.x. [DOI] [PubMed] [Google Scholar]
- 41.Kneib R T. Am Zool. 1986;26:259–269. [Google Scholar]
- 42.Hardy J D., Jr . Development of Fishes of the Mid-Atlantic Bight: An Atlas of Egg, Larval, and Juvenile Stages. Fort Collins, CO : U.S. Fish & Wildlife Service; 1978. USFWS Publ. No. FWS/OBS-78112. [Google Scholar]
- 43.Mitchell D L, Scoggins J T, Morizot D C. Photochem Photobiol. 1993;58:455–459. doi: 10.1111/j.1751-1097.1993.tb09590.x. [DOI] [PubMed] [Google Scholar]
- 44.Kellerman A, Kock K-H. In: Antarctic Ocean and Resources Variability. Sahrhage D, editor. Berlin: Springer; 1989. pp. 147–159. [Google Scholar]
- 45.Yasuihira S, Mitani H, Shima A. Photochem Photobiol. 1992;55:97–101. doi: 10.1111/j.1751-1097.1992.tb04214.x. [DOI] [PubMed] [Google Scholar]
- 46.Karentz D, Cleaver J E, Mitchell D L. J Phycol. 1991;27:326–341. [Google Scholar]
- 47.Mitchell D L, Hartman P S. BioEssays. 1990;12:74–79. doi: 10.1002/bies.950120205. [DOI] [PubMed] [Google Scholar]
- 48.El-Sayed S, editor. Southern Ocean Ecology. Cambridge, U.K.: Cambridge Univ. Press; 1994. [Google Scholar]
- 49.North A W. In: Biology of Antarctic Fish. di Prisco G, Maresca B, Tota B, editors. New York: Springer; 1991. pp. 70–86. [Google Scholar]
- 50.Morales-Nin B, Palomera I, Schadwinkel S. Polar Biol. 1995;15:143–154. [Google Scholar]
- 51.Targett T E. Mar Ecol Prog Ser. 1981;4:243. [Google Scholar]
- 52.Wormuth J H. Polar Biol. 1990;13:171–182. [Google Scholar]
- 53.Bidigare R R. Photochem Photobiol. 1989;50:469–477. [Google Scholar]
- 54.Hubolt G. In: Biology of Antarctic Fish. di Prisco G, Maresca B, Tota B, editors. New York: Springer; 1991. pp. 1–22. [Google Scholar]