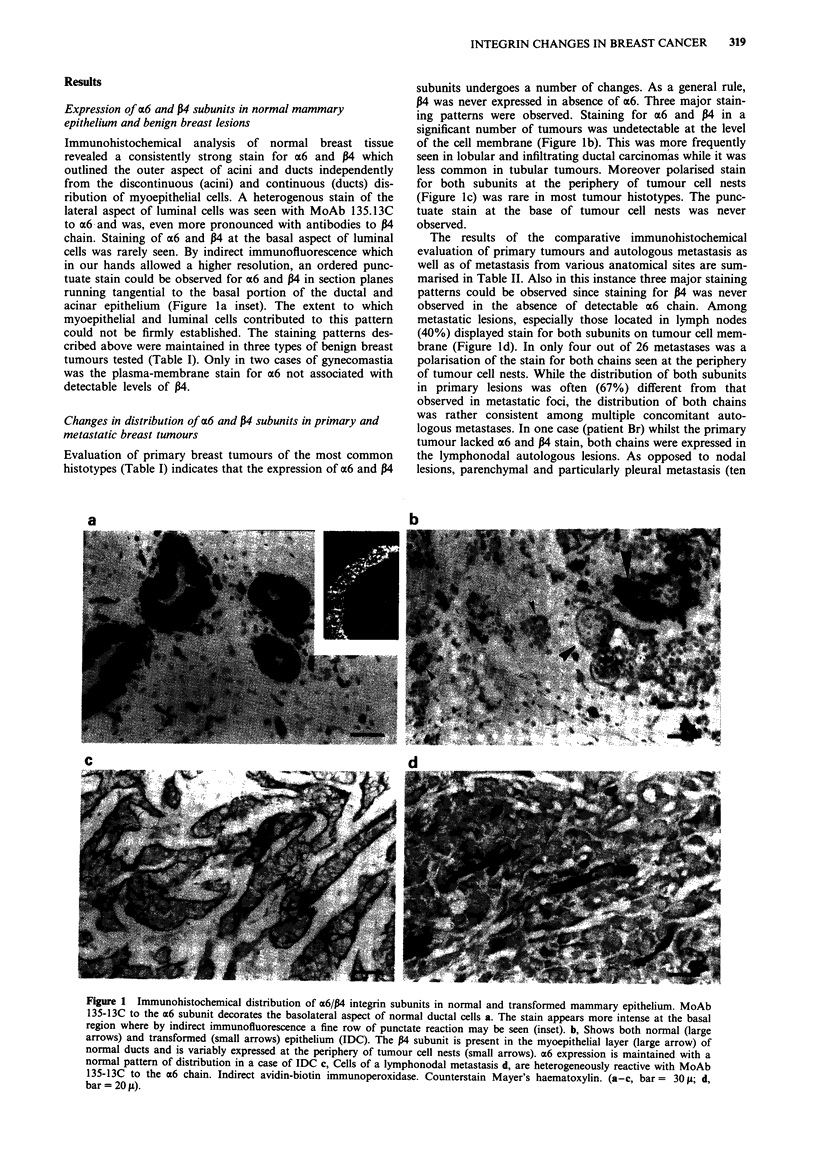
Abstract
The alpha 6/beta 4 integrin complex has been shown to be expressed in murine tissues at the basolateral aspect of most epithelial cells including the mammary epithelium, thus suggesting that this heterodimer may interact with components of the basement membrane. Because transformation of mammary epithelium frequently results in disappearance of basement membranes and loss of cell polarisation we have analysed in the present study whether expression of the alpha 6/beta 4 complex is altered in human breast tumours. The results of the present study confirm that in human mammary gland alpha 6 and beta 4 subunits colocalise at the basolateral aspect of the epithelium. While in benign breast lesions this distribution pattern remains mostly unchanged, in primary carcinomas the expression of both chains is either redistributed over the cells surface or significantly reduced. This altered pattern of expression is paralleled by the lack of detection of basement membrane laminin and collagen type IV. In metastatic lesions the expression of the heterodimer is maintained in most of the lymphnodal foci, but less frequently detected in metastasis localised in the pleural cavity and in parenchymal tissues. These findings indicate that in breast epithelium expression of the alpha 6/beta 4 heterodimer is modulated by the presence of basement membrane and is possibly influenced by microenvironmental factors as suggested by the different pattern of alpha 6/beta 4 expression in nodal and extranodal metastatic foci.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Albelda S. M., Mette S. A., Elder D. E., Stewart R., Damjanovich L., Herlyn M., Buck C. A. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990 Oct 15;50(20):6757–6764. [PubMed] [Google Scholar]
- Birembaut P., Caron Y., Adnet J. J., Foidart J. M. Usefulness of basement membrane markers in tumoural pathology. J Pathol. 1985 Apr;145(4):283–296. doi: 10.1002/path.1711450402. [DOI] [PubMed] [Google Scholar]
- Coopman P. J., Bracke M. E., Lissitzky J. C., De Bruyne G. K., Van Roy F. M., Foidart J. M., Mareel M. M. Influence of basement membrane molecules on directional migration of human breast cell lines in vitro. J Cell Sci. 1991 Mar;98(Pt 3):395–401. doi: 10.1242/jcs.98.3.395. [DOI] [PubMed] [Google Scholar]
- Dedhar S., Saulnier R. Alterations in integrin receptor expression on chemically transformed human cells: specific enhancement of laminin and collagen receptor complexes. J Cell Biol. 1990 Feb;110(2):481–489. doi: 10.1083/jcb.110.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcioni R., Kennel S. J., Giacomini P., Zupi G., Sacchi A. Expression of tumor antigen correlated with metastatic potential of Lewis lung carcinoma and B16 melanoma clones in mice. Cancer Res. 1986 Nov;46(11):5772–5778. [PubMed] [Google Scholar]
- Falcioni R., Sacchi A., Resau J., Kennel S. J. Monoclonal antibody to human carcinoma-associated protein complex: quantitation in normal and tumor tissue. Cancer Res. 1988 Feb 15;48(4):816–821. [PubMed] [Google Scholar]
- Fath K. R., Edgell C. J., Burridge K. The distribution of distinct integrins in focal contacts is determined by the substratum composition. J Cell Sci. 1989 Jan;92(Pt 1):67–75. doi: 10.1242/jcs.92.1.67. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990 Mar 9;60(5):849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Gould V. E., Jao W., Battifora H. Ultrastructural analysis in the differential diagnosis of breast tumors. The significance of myoepithelial cells, basal lamina, intracytoplasmic lumina and secretory granules. Pathol Res Pract. 1980 May;167(1):45–70. doi: 10.1016/S0344-0338(80)80181-6. [DOI] [PubMed] [Google Scholar]
- Heino J., Ignotz R. A., Hemler M. E., Crouse C., Massagué J. Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J Biol Chem. 1989 Jan 5;264(1):380–388. [PubMed] [Google Scholar]
- Hemler M. E., Crouse C., Sonnenberg A. Association of the VLA alpha 6 subunit with a novel protein. A possible alternative to the common VLA beta 1 subunit on certain cell lines. J Biol Chem. 1989 Apr 15;264(11):6529–6535. [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Ware C. F., Strominger J. L. Characterization of a novel differentiation antigen complex recognize by a monoclonal antibody (A-1A5): unique activation-specific molecular forms on stimulated T cells. J Immunol. 1983 Jul;131(1):334–340. [PubMed] [Google Scholar]
- Hirst R., Horwitz A., Buck C., Rohrschneider L. Phosphorylation of the fibronectin receptor complex in cells transformed by oncogenes that encode tyrosine kinases. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6470–6474. doi: 10.1073/pnas.83.17.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Kajiji S., Tamura R. N., Quaranta V. A novel integrin (alpha E beta 4) from human epithelial cells suggests a fourth family of integrin adhesion receptors. EMBO J. 1989 Mar;8(3):673–680. doi: 10.1002/j.1460-2075.1989.tb03425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen M., Ylänne J., Laitinen L., Virtanen I. The alpha 1-alpha 6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J Cell Biol. 1990 Sep;111(3):1245–1254. doi: 10.1083/jcb.111.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukoulis G. K., Virtanen I., Korhonen M., Laitinen L., Quaranta V., Gould V. E. Immunohistochemical localization of integrins in the normal, hyperplastic, and neoplastic breast. Correlations with their functions as receptors and cell adhesion molecules. Am J Pathol. 1991 Oct;139(4):787–799. [PMC free article] [PubMed] [Google Scholar]
- McGregor B. C., McGregor J. L., Weiss L. M., Wood G. S., Hu C. H., Boukerche H., Warnke R. A. Presence of cytoadhesins (IIb-IIIa-like glycoproteins) on human metastatic melanomas but not on benign melanocytes. Am J Clin Pathol. 1989 Oct;92(4):495–499. doi: 10.1093/ajcp/92.4.495. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Imai K., Wilson B. S., Bigotti A., Cavaliere R., Pellegrino M. A., Ferrone S. Structural properties and tissue distribution of the antigen recognized by the monoclonal antibody 653.40S to human melanoma cells. J Natl Cancer Inst. 1981 Sep;67(3):591–601. [PubMed] [Google Scholar]
- Natali P. G., Nicotra M. R., Cavaliere R., Giannarelli D., Bigotti A. Tumor progression in human malignant melanoma is associated with changes in alpha 6/beta 1 laminin receptor. Int J Cancer. 1991 Sep 9;49(2):168–172. doi: 10.1002/ijc.2910490203. [DOI] [PubMed] [Google Scholar]
- Plantefaber L. C., Hynes R. O. Changes in integrin receptors on oncogenically transformed cells. Cell. 1989 Jan 27;56(2):281–290. doi: 10.1016/0092-8674(89)90902-1. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Giancotti F. G. Integrins and tumor cell dissemination. Cancer Cells. 1989 Dec;1(4):119–126. [PubMed] [Google Scholar]
- Sonnenberg A., Hogervorst F., Osterop A., Veltman F. E. Identification and characterization of a novel antigen complex on mouse mammary tumor cells using a monoclonal antibody against platelet glycoprotein Ic. J Biol Chem. 1988 Oct 5;263(28):14030–14038. [PubMed] [Google Scholar]
- Sonnenberg A., Janssen H., Hogervorst F., Calafat J., Hilgers J. A complex of platelet glycoproteins Ic and IIa identified by a rat monoclonal antibody. J Biol Chem. 1987 Jul 25;262(21):10376–10383. [PubMed] [Google Scholar]
- Sonnenberg A., Linders C. J., Daams J. H., Kennel S. J. The alpha 6 beta 1 (VLA-6) and alpha 6 beta 4 protein complexes: tissue distribution and biochemical properties. J Cell Sci. 1990 Jun;96(Pt 2):207–217. doi: 10.1242/jcs.96.2.207. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Modderman P. W., Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988 Dec 1;336(6198):487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Streuli C. H., Bailey N., Bissell M. J. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991 Dec;115(5):1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubura A., Shikata N., Inui T., Morii S., Hatano T., Oikawa T., Matsuzawa A. Immunohistochemical localization of myoepithelial cells and basement membrane in normal, benign and malignant human breast lesions. Virchows Arch A Pathol Anat Histopathol. 1988;413(2):133–139. doi: 10.1007/BF00749674. [DOI] [PubMed] [Google Scholar]