Abstract
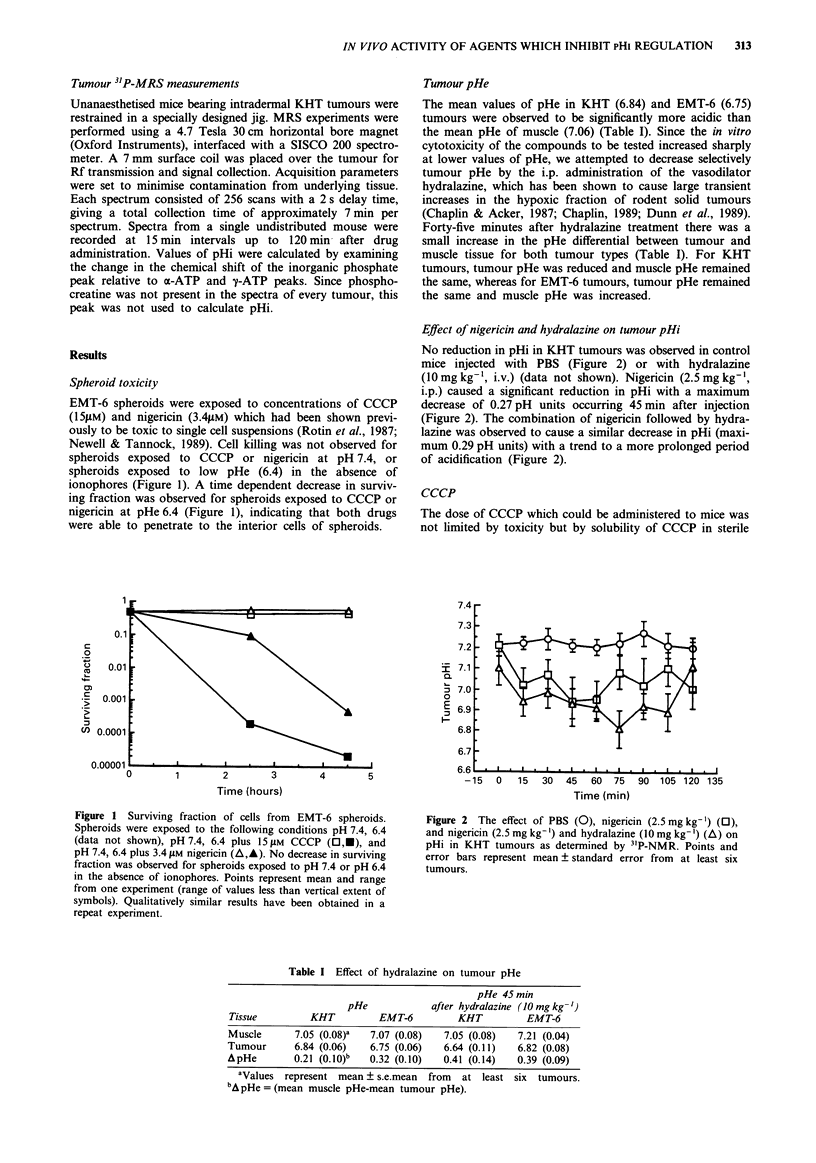
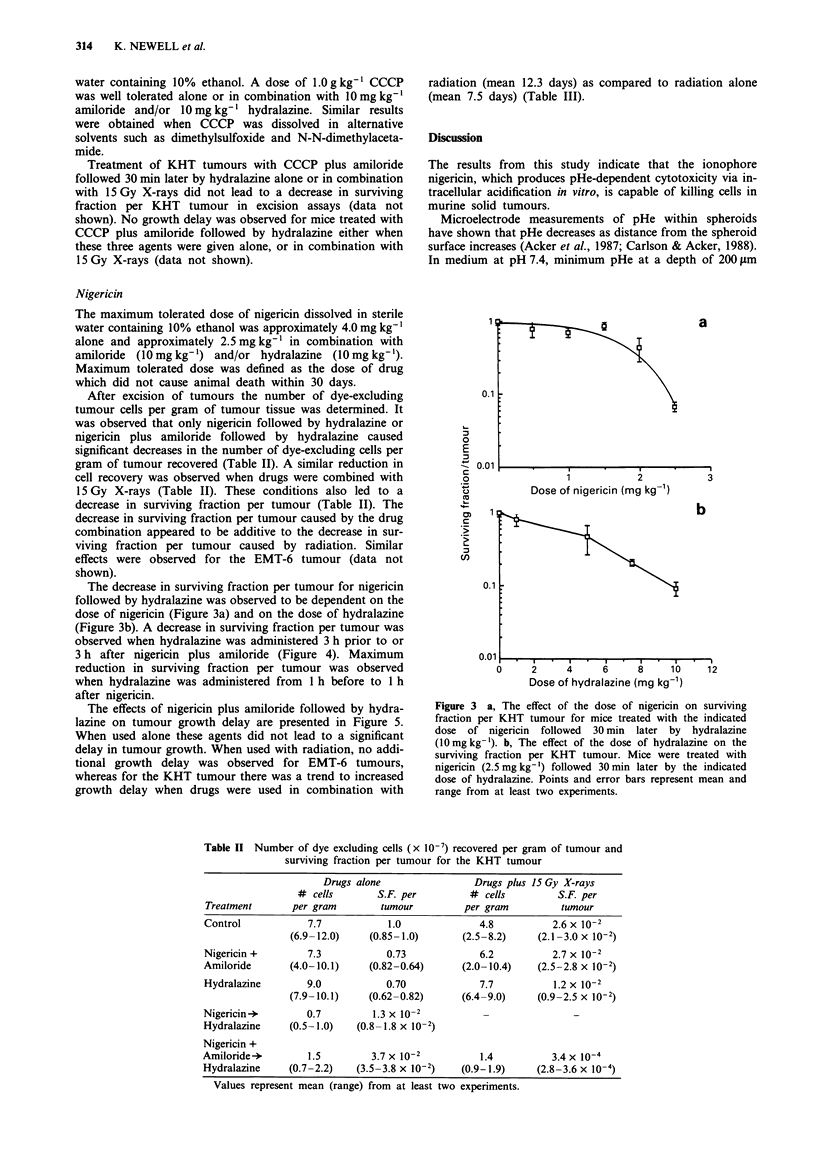
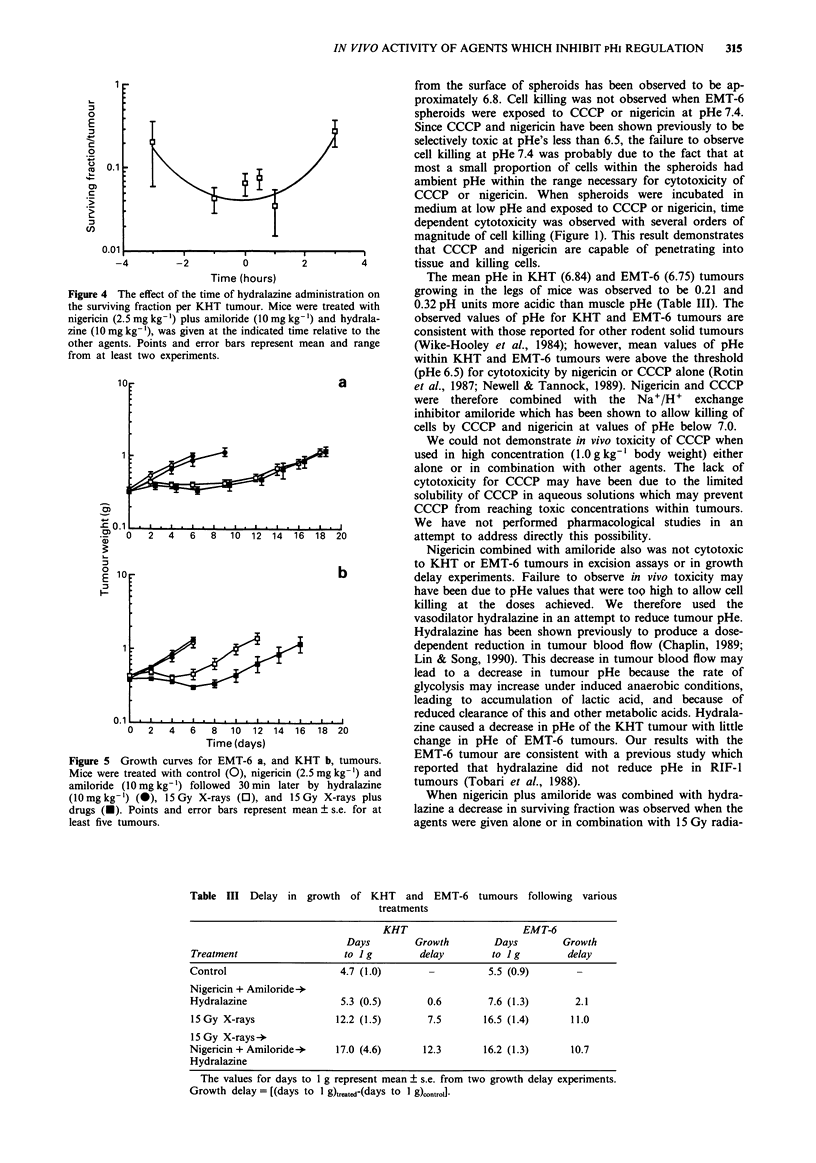
Cell killing can be achieved in an acidic environment in tissue culture (medium pH less than 7.0) by agents (nigericin, carbonylcyanide-3-chlorophenylhydrazone (CCCP)) which transport protons from the extracellular space into the cytoplasm. Cell killing is enhanced when these agents are used in combination with compounds (amiloride, 4,4'-diisothiocyanostilbene-2,2'-disulfonic acid (DIDS)) which inhibit the membrane-based exchangers responsible for the regulation of intracellular pH (pHi). We describe experiments which assess the ability of these agents to kill tumour cells in spheroids and in vivo. Both nigericin and CCCP were observed to penetrate tissue based on their ability to kill tumour cells in spheroids. The mean extracellular pH (pHe) of the KHT fibrosarcoma and the EMT-6 sarcoma were observed to be 0.21 and 0.32 pH units more acidic than the mean pHe in muscle tissue. Intraperitoneal (i.p.) administration of the vasodilator hydralazine (10 mg kg-1) caused a reduction of the mean pHe of the KHT but not the EMT-6 tumour. Nigericin (2.5 mg kg-1, i.p.) plus amiloride (10 mg kg-1, i.p.) followed 30 min later by hydralazine (10 mg kg-1, i.p.) reduced the surviving fraction of cells in the KHT and EMT-6 tumours, but had minimal effects on growth delay. When KHT tumours were treated with 15 Gy X-rays followed immediately by nigericin plus amiloride and hydralazine a reduced surviving fraction as well as an increase in tumour growth delay was observed compared to radiation alone. The administration of nigericin (2.5 mg kg-1, i.p.) or the combination of nigericin (2.5 mg kg-1, i.p.) followed by hydralazine (10 mg kg-1, intravenous (i.v.)) resulted in reductions of tumour pHi of 0.27 and 0.29 pH units respectively as determined by 31P magnetic resonance spectroscopy (MRS). Our results show that the combination of nigericin and hydralazine (with or without amiloride) can kill cells in rodent solid tumours and that cell killing is associated with a reduction in the mean pHi of tumour cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker H., Carlsson J., Holtermann G., Nederman T., Nylén T. Influence of glucose and buffer capacity in the culture medium on growth and pH in spheroids of human thyroid carcinoma and human glioma origin. Cancer Res. 1987 Jul 1;47(13):3504–3508. [PubMed] [Google Scholar]
- Busa W. B., Nuccitelli R. Metabolic regulation via intracellular pH. Am J Physiol. 1984 Apr;246(4 Pt 2):R409–R438. doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Acker H. Relations between pH, oxygen partial pressure and growth in cultured cell spheroids. Int J Cancer. 1988 Nov 15;42(5):715–720. doi: 10.1002/ijc.2910420515. [DOI] [PubMed] [Google Scholar]
- Cassel D., Scharf O., Rotman M., Cragoe E. J., Jr, Katz M. Characterization of Na+-linked and Na+-independent Cl-/HCO3- exchange systems in Chinese hamster lung fibroblasts. J Biol Chem. 1988 May 5;263(13):6122–6127. [PubMed] [Google Scholar]
- Chaplin D. J., Acker B. The effect of hydralazine on the tumor cytotoxicity of the hypoxic cell cytotoxin RSU-1069: evidence for therapeutic gain. Int J Radiat Oncol Biol Phys. 1987 Apr;13(4):579–585. doi: 10.1016/0360-3016(87)90075-7. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Durand R. E., Olive P. L. Cell selection from a murine tumour using the fluorescent probe Hoechst 33342. Br J Cancer. 1985 Apr;51(4):569–572. doi: 10.1038/bjc.1985.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D. J. Hydralazine-induced tumor hypoxia: a potential target for cancer chemotherapy. J Natl Cancer Inst. 1989 Apr 19;81(8):618–622. doi: 10.1093/jnci/81.8.618. [DOI] [PubMed] [Google Scholar]
- Dobrowsky E., Newell K., Tannock I. F. The potential of lactate and succinate to kill nutrient deprived tumor cells by intracellular acidification. Int J Radiat Oncol Biol Phys. 1991 Feb;20(2):275–279. doi: 10.1016/0360-3016(91)90104-c. [DOI] [PubMed] [Google Scholar]
- Dunn J. F., Frostick S., Adams G. E., Stratford I. J., Howells N., Hogan G., Radda G. K. Induction of tumour hypoxia by a vasoactive agent. A combined NMR and radiobiological study. FEBS Lett. 1989 Jun 5;249(2):343–347. doi: 10.1016/0014-5793(89)80655-6. [DOI] [PubMed] [Google Scholar]
- Durand R. E. Use of Hoechst 33342 for cell selection from multicell systems. J Histochem Cytochem. 1982 Feb;30(2):117–122. doi: 10.1177/30.2.6174559. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rotin D., Mason M. J. Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim Biophys Acta. 1989 Jan 18;988(1):73–97. doi: 10.1016/0304-4157(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Song C. W. Effects of hydralazine on the blood flow in RIF-1 tumors and normal tissues of mice. Radiat Res. 1990 Nov;124(2):171–177. [PubMed] [Google Scholar]
- Minchinton A. I., Durand R. E., Chaplin D. J. Intermittent blood flow in the KHT sarcoma--flow cytometry studies using Hoechst 33342. Br J Cancer. 1990 Aug;62(2):195–200. doi: 10.1038/bjc.1990.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. E., Rockwell S. Hypoxic fractions of solid tumors: experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys. 1984 May;10(5):695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- Newell K. J., Tannock I. F. Reduction of intracellular pH as a possible mechanism for killing cells in acidic regions of solid tumors: effects of carbonylcyanide-3-chlorophenylhydrazone. Cancer Res. 1989 Aug 15;49(16):4477–4482. [PubMed] [Google Scholar]
- Reinertsen K. V., Tønnessen T. I., Jacobsen J., Sandvig K., Olsnes S. Role of chloride/bicarbonate antiport in the control of cytosolic pH. Cell-line differences in activity and regulation of antiport. J Biol Chem. 1988 Aug 15;263(23):11117–11125. [PubMed] [Google Scholar]
- Rotin D., Steele-Norwood D., Grinstein S., Tannock I. Requirement of the Na+/H+ exchanger for tumor growth. Cancer Res. 1989 Jan 1;49(1):205–211. [PubMed] [Google Scholar]
- Rotin D., Wan P., Grinstein S., Tannock I. Cytotoxicity of compounds that interfere with the regulation of intracellular pH: a potential new class of anticancer drugs. Cancer Res. 1987 Mar 15;47(6):1497–1504. [PubMed] [Google Scholar]
- THOMLINSON R. H., GRAY L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955 Dec;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I. F., Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989 Aug 15;49(16):4373–4384. [PubMed] [Google Scholar]
- Tannock I. F. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer. 1968 Jun;22(2):258–273. doi: 10.1038/bjc.1968.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I. Response of aerobic and hypoxic cells in a solid tumor to adriamycin and cyclophosphamide and interaction of the drugs with radiation. Cancer Res. 1982 Dec;42(12):4921–4926. [PubMed] [Google Scholar]
- Thomson J. E., Rauth A. M. An in vitro assay to measure the viability of KHT tumor cells not previously exposed to culture conditions. Radiat Res. 1974 May;58(2):262–276. [PubMed] [Google Scholar]
- Tobari C., Van Kersen I., Hahn G. M. Modification of pH of normal and malignant mouse tissue by hydralazine and glucose, with and without breathing of 5% CO2 and 95% air. Cancer Res. 1988 Mar 15;48(6):1543–1547. [PubMed] [Google Scholar]
- Trivedi B., Danforth W. H. Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem. 1966 Sep 10;241(17):4110–4112. [PubMed] [Google Scholar]
- Varnes M. E., Glazier K. G., Gray C. pH-dependent effects of the ionophore nigericin on response of mammalian cells to radiation and heat treatment. Radiat Res. 1989 Feb;117(2):282–292. [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Whillans D. W., Rauth A. M. An experimental and analytical study of oxygen depletion in stirred cell suspensions. Radiat Res. 1980 Oct;84(1):97–114. [PubMed] [Google Scholar]
- Wike-Hooley J. L., Haveman J., Reinhold H. S. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984 Dec;2(4):343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]



