Abstract
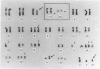
We have investigated the capability of differentiation of Ewing's sarcoma (ES) towards a neuronal direction through the establishment of four extraosseous ES cell lines and by in vitro stimulation with dibutyryladenosine cyclic monophosphate (db-cAMP) of eight ES lines. All except one of the lines expressed the molecule defined by 5C11, the antibody specifically reactive with ES. Two ES lines expressed a 200 kilodalton (kD) neurofilament protein (NFP) although their original tumours were negative for NFP. Elongation of cytoplasmic processes and increased NFP expression were observed after db-cAMP treatment of these lines and microtubules in the cytoplasmic processes were ultrastructurally demonstrated. Six lines were NFP negative, but three lines changed their morphology after induction of 200 kD NFP expression by db-cAMP treatment. The other three showed no definitive differentiation after db-cAMP treatment. Chromosomal analysis of the new ES lines showed the typical t(11;22) in one line and a +der(22) in two lines. No correlation was observed between the chromosomal abnormality and the differentiation capability. We conclude that ES is a heterogeneous group of tumours with respect to capability of differentiation into the neuronal lineage, but it is clearly distinguished from peripheral primitive neuroectodermal tumours by its 5C11 reactivity.
Full text
PDF

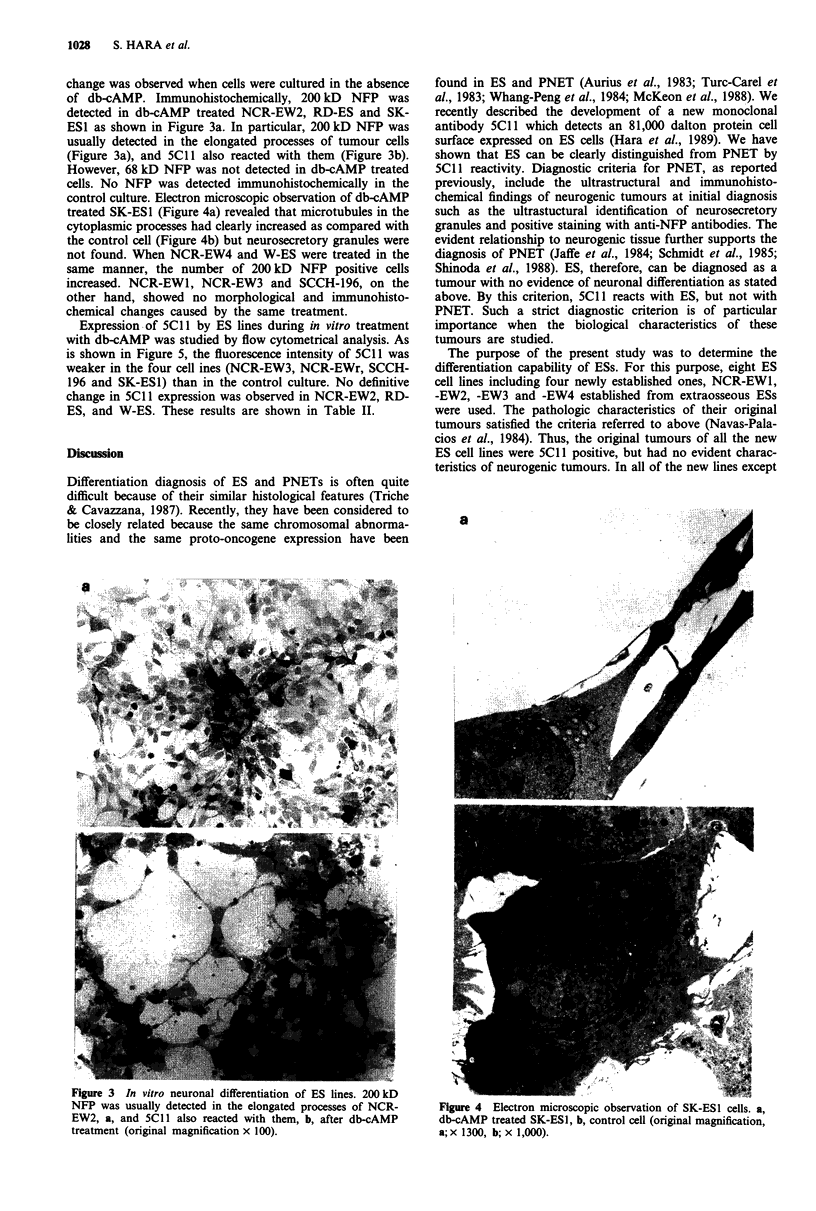
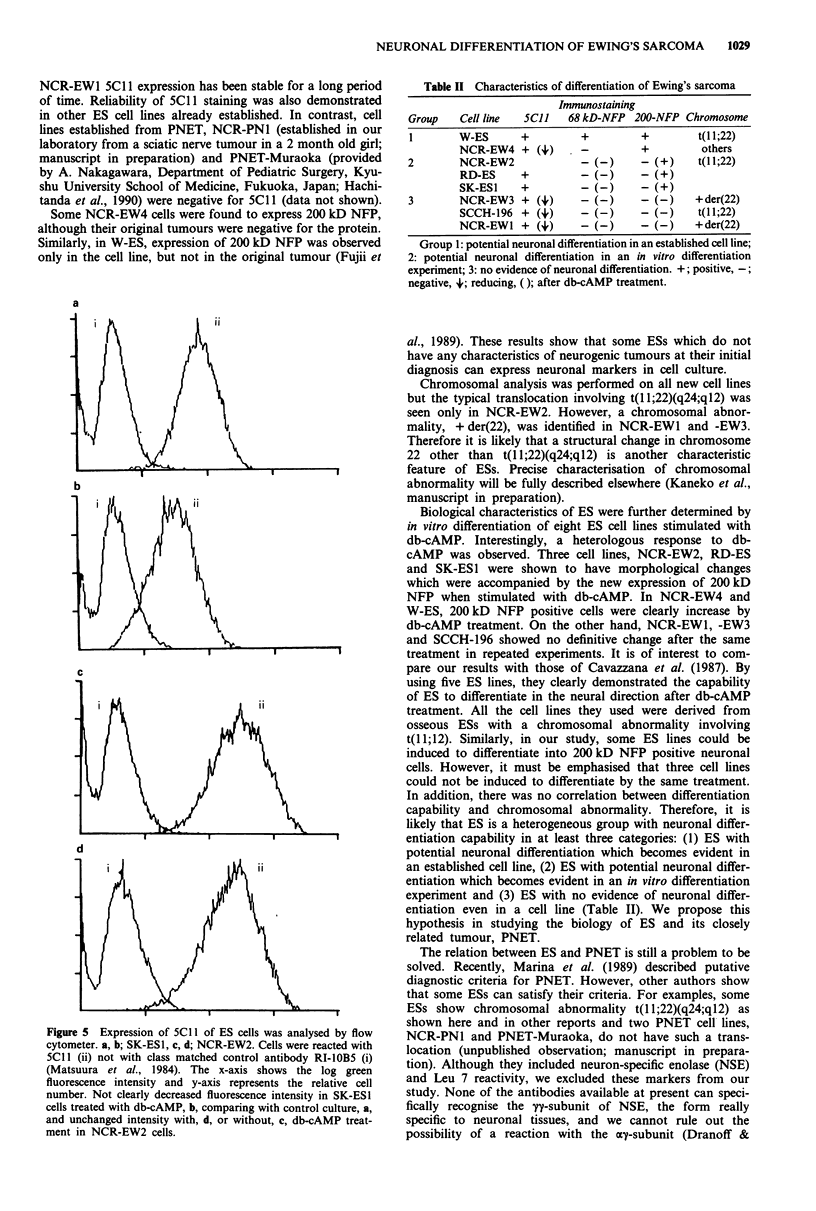

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Abo T., Cooper M. D., Balch C. M. Postnatal expansion of the natural killer and keller cell population in humans identified by the monoclonal HNK-1 antibody. J Exp Med. 1982 Jan 1;155(1):321–326. doi: 10.1084/jem.155.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angervall L., Enzinger F. M. Extraskeletal neoplasm resembling Ewing's sarcoma. Cancer. 1975 Jul;36(1):240–251. doi: 10.1002/1097-0142(197507)36:1<240::aid-cncr2820360127>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Cavazzana A. O., Miser J. S., Jefferson J., Triche T. J. Experimental evidence for a neural origin of Ewing's sarcoma of bone. Am J Pathol. 1987 Jun;127(3):507–518. [PMC free article] [PubMed] [Google Scholar]
- Dehner L. P. Peripheral and central primitive neuroectodermal tumors. A nosologic concept seeking a consensus. Arch Pathol Lab Med. 1986 Nov;110(11):997–1005. [PubMed] [Google Scholar]
- Dranoff G., Bigner D. D. A word of caution in the use of neuron-specific enolase expression in tumor diagnosis. Arch Pathol Lab Med. 1984 Jul;108(7):535–535. [PubMed] [Google Scholar]
- Hachitanda Y., Tsuneyoshi M., Enjoji M., Nakagawara A., Ikeda K. Congenital primitive neuroectodermal tumor with epithelial and glial differentiation. An ultrastructural and immunohistochemical study. Arch Pathol Lab Med. 1990 Jan;114(1):101–105. [PubMed] [Google Scholar]
- Hara S., Ishii E., Tanaka S., Yokoyama J., Katsumata K., Fujimoto J., Hata J. A monoclonal antibody specifically reactive with Ewing's sarcoma. Br J Cancer. 1989 Dec;60(6):875–879. doi: 10.1038/bjc.1989.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata J. I., Ueyama Y., Tamaoki N., Akatsuka A., Yoshimura S., Shimuzu K., Morikawa Y., Furukawa T. Human yolk sac tumor serially transplanted in nude mice: its morphologic and functional properties. Cancer. 1980 Dec 1;46(11):2446–2455. doi: 10.1002/1097-0142(19801201)46:11<2446::aid-cncr2820461125>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Homma C., Kaneko Y., Sekine K., Hara S., Hata J., Sakurai M. Establishment and characterization of a small round cell sarcoma cell line, SCCH-196, with t(11;22)(q24;q12). Jpn J Cancer Res. 1989 Sep;80(9):861–865. doi: 10.1111/j.1349-7006.1989.tb01727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe R., Santamaria M., Yunis E. J., Tannery N. H., Agostini R. M., Jr, Medina J., Goodman M. The neuroectodermal tumor of bone. Am J Surg Pathol. 1984 Dec;8(12):885–898. doi: 10.1097/00000478-198412000-00001. [DOI] [PubMed] [Google Scholar]
- Jürgens H., Bier V., Harms D., Beck J., Brandeis W., Etspüler G., Gadner H., Schmidt D., Treuner J., Winkler K. Malignant peripheral neuroectodermal tumors. A retrospective analysis of 42 patients. Cancer. 1988 Jan 15;61(2):349–357. doi: 10.1002/1097-0142(19880115)61:2<349::aid-cncr2820610226>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Marina N. M., Etcubanas E., Parham D. M., Bowman L. C., Green A. Peripheral primitive neuroectodermal tumor (peripheral neuroepithelioma) in children. A review of the St. Jude experience and controversies in diagnosis and management. Cancer. 1989 Nov 1;64(9):1952–1960. doi: 10.1002/1097-0142(19891101)64:9<1952::aid-cncr2820640931>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Matsuura A., Ishii Y., Yuasa H., Narita H., Kon S., Takami T., Kikuchi K. Rat T lymphocyte antigens comparable with mouse Lyt-1 and Lyt-2,3 antigenic systems: characterization by monoclonal antibodies. J Immunol. 1984 Jan;132(1):316–322. [PubMed] [Google Scholar]
- McKeon C., Thiele C. J., Ross R. A., Kwan M., Triche T. J., Miser J. S., Israel M. A. Indistinguishable patterns of protooncogene expression in two distinct but closely related tumors: Ewing's sarcoma and neuroepithelioma. Cancer Res. 1988 Aug 1;48(15):4307–4311. [PubMed] [Google Scholar]
- Navas-Palacios J. J., Aparicio-Duque R., Valdés M. D. On the histogenesis of Ewing's sarcoma. An ultrastructural, immunohistochemical, and cytochemical study. Cancer. 1984 May 1;53(9):1882–1901. doi: 10.1002/1097-0142(19840501)53:9<1882::aid-cncr2820530915>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Schmechel D. E. Gamma-subunit of the glycolytic enzyme enolase: nonspecific or neuron specific? Lab Invest. 1985 Mar;52(3):239–242. [PubMed] [Google Scholar]
- Shimada H., Newton W. A., Jr, Soule E. H., Qualman S. J., Aoyama C., Maurer H. M. Pathologic features of extraosseous Ewing's sarcoma: a report from the Intergroup Rhabdomyosarcoma Study. Hum Pathol. 1988 Apr;19(4):442–453. doi: 10.1016/s0046-8177(88)80495-7. [DOI] [PubMed] [Google Scholar]
- Shinoda M., Tsutsumi Y., Hata J., Yokoyama S. Peripheral neuroepithelioma in childhood. Immunohistochemical demonstration of epithelial differentiation. Arch Pathol Lab Med. 1988 Nov;112(11):1155–1158. [PubMed] [Google Scholar]
- Tefft M., Vawter G. F., Mitus A. Paravertebral "round cell" tumors in children. Radiology. 1969 Jun;92(7):1501–1509. doi: 10.1148/92.7.1501. [DOI] [PubMed] [Google Scholar]
- Vinores S. A., Bonnin J. M., Rubinstein L. J., Marangos P. J. Immunohistochemical demonstration of neuron-specific enolase in neoplasms of the CNS and other tissues. Arch Pathol Lab Med. 1984 Jul;108(7):536–540. [PubMed] [Google Scholar]
- Voss B. L., Pysher T. J., Humphrey G. B. Peripheral neuroepithelioma in childhood. Cancer. 1984 Dec 15;54(12):3059–3064. doi: 10.1002/1097-0142(19841215)54:12<3059::aid-cncr2820541241>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Whang-Peng J., Triche T. J., Knutsen T., Miser J., Douglass E. C., Israel M. A. Chromosome translocation in peripheral neuroepithelioma. N Engl J Med. 1984 Aug 30;311(9):584–585. doi: 10.1056/NEJM198408303110907. [DOI] [PubMed] [Google Scholar]