Abstract
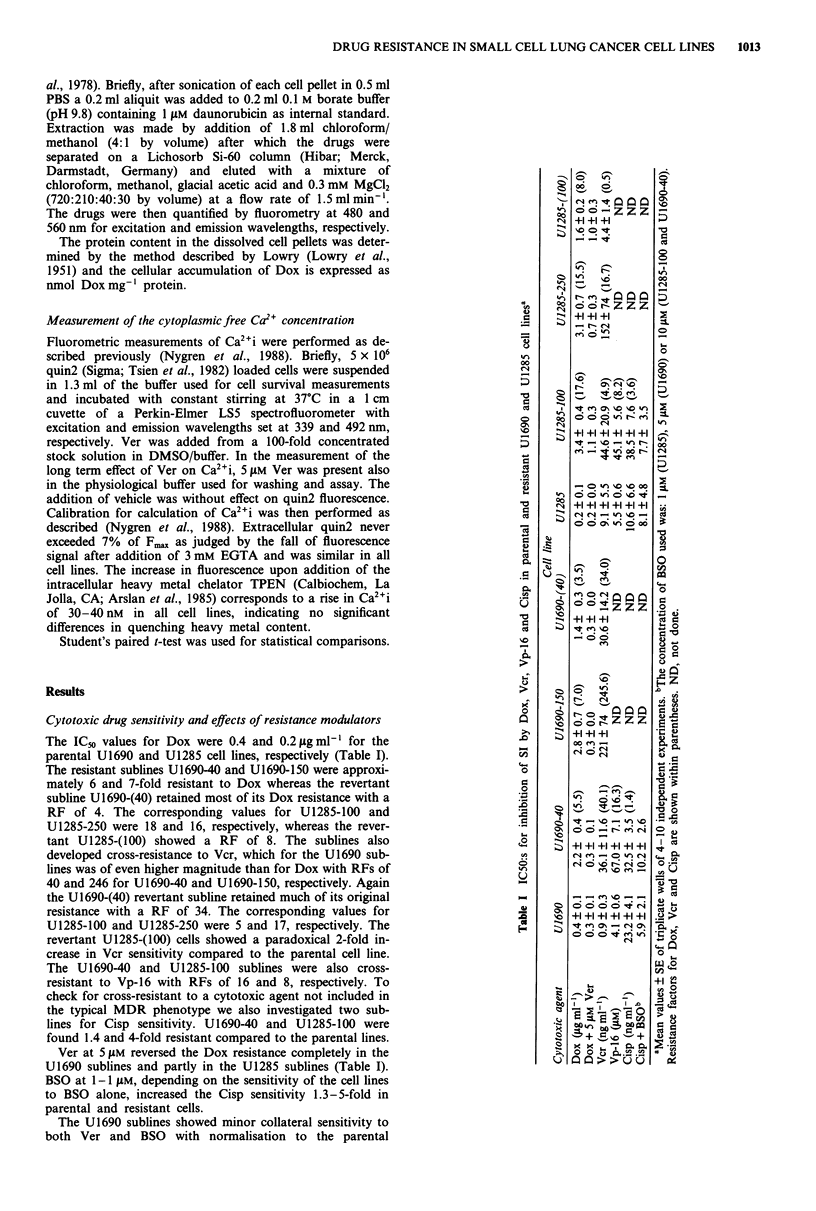
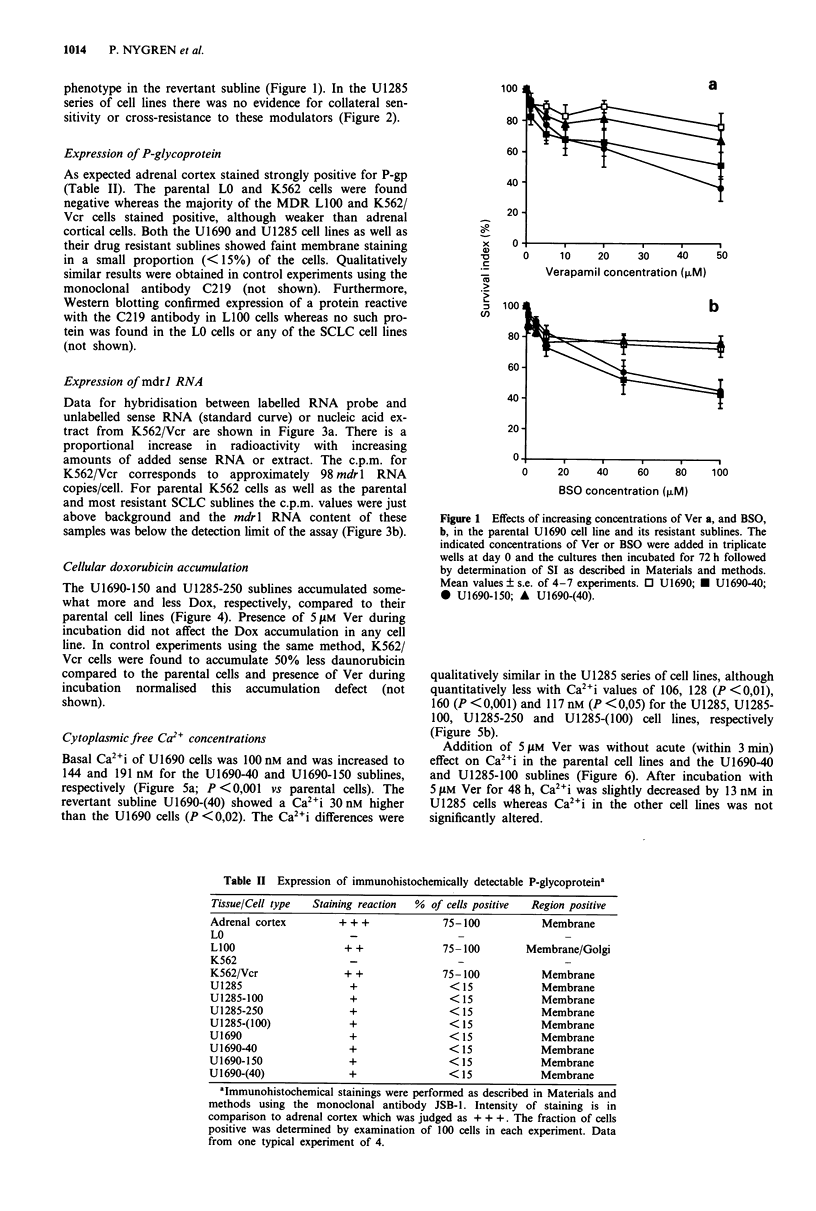
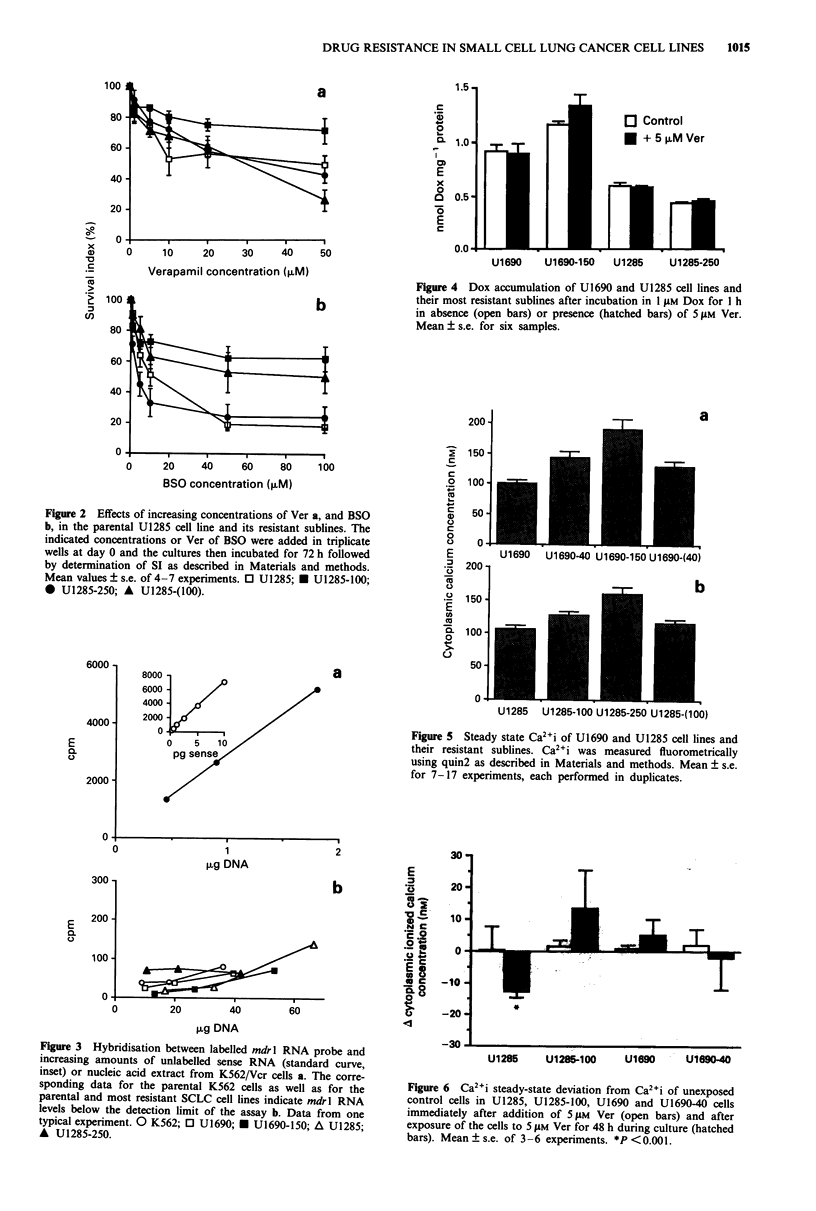
Sublines from the small cell lung cancer (SCLC) cell lines U1285 and U1690, denoted U1285-100, U1285-250, U1690-40 and U1690-150, were adapted to grow in the continuous presence of 100, 250, 40 and 150 ng ml-1 doxorubicin (Dox), respectively. The Dox resistance was accompanied by cross-resistance to vincristine (Vcr), Vp-16 and for U1285-100 also to cisplatinum. Sublines of U1690-40 and U1285-100, cultured in absence of Dox for 4 months were only partially reversed with respect to Dox resistance. Neither the parental nor the most Dox resistance sublines had detectable levels of mdr 1 RNA but a small fraction of cells in all cell lines stained weakly positive for P-glycoprotein (P-gp). Verapamil (Ver) at 5 microM reversed the Dox resistance completely and partly in the U1690 and U1285 sublines, respectively, but did not increase the cellular accumulation of Dox. The cytoplasmic free Ca2+ concentration (Ca2+i) was close to 100 nM in both parental cell lines but elevated in the U1285-100 and U1690-40 sublines by 21 and 44%, respectively, and in U1285-250 and U1690-150 by 51 and 91%, respectively. The partly reverted sublines still showed significant but smaller elevations in Ca2+i of 10-30%. Ver was without acute or long term effects of Ca2+i in the U1285-100 and U1690-40 sublines. Selection for Dox resistance in SCLC may thus result in atypical multidrug-resistance characterised by absence of P-gp overexpression and atypical cross-resistance. Although Ver did not seem to affect Dox accumulation it may still work as a resistance modulator.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arslan P., Di Virgilio F., Beltrame M., Tsien R. Y., Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem. 1985 Mar 10;260(5):2719–2727. [PubMed] [Google Scholar]
- Baas F., Jongsma A. P., Broxterman H. J., Arceci R. J., Housman D., Scheffer G. L., Riethorst A., van Groenigen M., Nieuwint A. W., Joenje H. Non-P-glycoprotein mediated mechanism for multidrug resistance precedes P-glycoprotein expression during in vitro selection for doxorubicin resistance in a human lung cancer cell line. Cancer Res. 1990 Sep 1;50(17):5392–5398. [PubMed] [Google Scholar]
- Baurain R., Zenebergh A., Trouet A. Cellular uptake and metabolism of daunorubicin as determined by high-performance liquid chromatography. Application to L1210 cells. J Chromatogr. 1978 Sep 21;157:331–336. doi: 10.1016/s0021-9673(00)92350-1. [DOI] [PubMed] [Google Scholar]
- Beck W. T., Cirtain M. C., Danks M. K., Felsted R. L., Safa A. R., Wolverton J. S., Suttle D. P., Trent J. M. Pharmacological, molecular, and cytogenetic analysis of "atypical" multidrug-resistant human leukemic cells. Cancer Res. 1987 Oct 15;47(20):5455–5460. [PubMed] [Google Scholar]
- Beck W. T. The cell biology of multiple drug resistance. Biochem Pharmacol. 1987 Sep 15;36(18):2879–2887. doi: 10.1016/0006-2952(87)90198-5. [DOI] [PubMed] [Google Scholar]
- Bergh J., Esscher T., Steinholtz L., Nilsson K., Påhlman S. Immunocytochemical demonstration of neuron-specific enolase (NSE) in human lung cancers. Am J Clin Pathol. 1985 Jul;84(1):1–7. doi: 10.1093/ajcp/84.1.1. [DOI] [PubMed] [Google Scholar]
- Bergh J., Larsson E., Zech L., Nilsson K. Establishment and characterization of two neoplastic cell lines (U-1285 and U-1568) derived from small cell carcinoma of the lung. Acta Pathol Microbiol Immunol Scand A. 1982 May;90(3):149–158. doi: 10.1111/j.1699-0463.1982.tb00076_90a.x. [DOI] [PubMed] [Google Scholar]
- Bergh J., Nilsson K., Ekman R., Giovanella B. Establishment and characterization of cell lines from human small cell and large cell carcinomas of the lung. Acta Pathol Microbiol Immunol Scand A. 1985 May;93(3):133–147. doi: 10.1111/j.1699-0463.1985.tb03932.x. [DOI] [PubMed] [Google Scholar]
- Bradley G., Juranka P. F., Ling V. Mechanism of multidrug resistance. Biochim Biophys Acta. 1988 Aug 3;948(1):87–128. doi: 10.1016/0304-419x(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Caroni P., Villani F., Carafoli E. The cardiotoxic antibiotic doxorubicin inhibits the Na+/Ca2+ exchange of dog heart sarcolemmal vesicles. FEBS Lett. 1981 Aug 3;130(2):184–186. doi: 10.1016/0014-5793(81)81115-5. [DOI] [PubMed] [Google Scholar]
- Chang B. K., Brenner D. E., Gutman R. Dissociation of the verapamil-induced enhancement of doxorubicin's cytotoxicity from changes in cellular accumulation or retention of doxorubicin in pancreatic cancer cell lines. Anticancer Res. 1989 Mar-Apr;9(2):347–351. [PubMed] [Google Scholar]
- Cole S. P., Downes H. F., Slovak M. L. Effect of calcium antagonists on the chemosensitivity of two multidrug-resistant human tumour cell lines which do not overexpress P-glycoprotein. Br J Cancer. 1989 Jan;59(1):42–46. doi: 10.1038/bjc.1989.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton W. S., Grogan T. M., Rybski J. A., Scheper R. J., Richter L., Kailey J., Broxterman H. J., Pinedo H. M., Salmon S. E. Immunohistochemical detection and quantitation of P-glycoprotein in multiple drug-resistant human myeloma cells: association with level of drug resistance and drug accumulation. Blood. 1989 Feb 15;73(3):747–752. [PubMed] [Google Scholar]
- Deffie A. M., Alam T., Seneviratne C., Beenken S. W., Batra J. K., Shea T. C., Henner W. D., Goldenberg G. J. Multifactorial resistance to adriamycin: relationship of DNA repair, glutathione transferase activity, drug efflux, and P-glycoprotein in cloned cell lines of adriamycin-sensitive and -resistant P388 leukemia. Cancer Res. 1988 Jul 1;48(13):3595–3602. [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. A practical approach for quantitating specific mRNAs by solution hybridization. Anal Biochem. 1983 Jun;131(2):385–393. doi: 10.1016/0003-2697(83)90188-4. [DOI] [PubMed] [Google Scholar]
- Goldstein L. J., Galski H., Fojo A., Willingham M., Lai S. L., Gazdar A., Pirker R., Green A., Crist W., Brodeur G. M. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst. 1989 Jan 18;81(2):116–124. doi: 10.1093/jnci/81.2.116. [DOI] [PubMed] [Google Scholar]
- Haber M., Norris M. D., Kavallaris M., Bell D. R., Davey R. A., White L., Stewart B. W. Atypical multidrug resistance in a therapy-induced drug-resistant human leukemia cell line (LALW-2): resistance to Vinca alkaloids independent of P-glycoprotein. Cancer Res. 1989 Oct 1;49(19):5281–5287. [PubMed] [Google Scholar]
- Horio M., Gottesman M. M., Pastan I. ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proc Natl Acad Sci U S A. 1988 May;85(10):3580–3584. doi: 10.1073/pnas.85.10.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet S., Robert J. The reversal of doxorubicin resistance by verapamil is not due to an effect on calcium channels. Int J Cancer. 1988 Feb 15;41(2):283–286. doi: 10.1002/ijc.2910410220. [DOI] [PubMed] [Google Scholar]
- Kartner N., Evernden-Porelle D., Bradley G., Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. 1985 Aug 29-Sep 4Nature. 316(6031):820–823. doi: 10.1038/316820a0. [DOI] [PubMed] [Google Scholar]
- Keyes S. R., Hickman J. A., Sartorelli A. C. The effects of adriamycin on intracellular calcium concentrations of L1210 murine leukemia cells. Eur J Cancer Clin Oncol. 1987 Mar;23(3):295–302. doi: 10.1016/0277-5379(87)90073-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Larsson R., Nygren P. Pharmacological modification of multi-drug resistance (MDR) in vitro detected by a novel fluorometric microculture cytotoxicity assay. Reversal of resistance and selective cytotoxic actions of cyclosporin A and verapamil on MDR leukemia T-cells. Int J Cancer. 1990 Jul 15;46(1):67–72. doi: 10.1002/ijc.2910460114. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Mathews L. S., Norstedt G., Palmiter R. D. Regulation of insulin-like growth factor I gene expression by growth hormone. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9343–9347. doi: 10.1073/pnas.83.24.9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath T., Center M. S. Mechanisms of multidrug resistance in HL60 cells: evidence that a surface membrane protein distinct from P-glycoprotein contributes to reduced cellular accumulation of drug. Cancer Res. 1988 Jul 15;48(14):3959–3963. [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988 Nov 25;263(33):17205–17208. [PubMed] [Google Scholar]
- Morgan S. A., Watson J. V., Twentyman P. R., Smith P. J. Flow cytometric analysis of Hoechst 33342 uptake as an indicator of multi-drug resistance in human lung cancer. Br J Cancer. 1989 Sep;60(3):282–287. doi: 10.1038/bjc.1989.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S., Samy T. S., Krishan A. Calcium, calmodulin, and protein content of adriamycin-resistant and -sensitive murine leukemic cells. Cancer Res. 1986 Jan;46(1):229–232. [PubMed] [Google Scholar]
- Nygren P., Gylfe E., Larsson R., Johansson H., Juhlin C., Klareskoq L., Akerström G., Rastad J. Modulation of the Ca2+-sensing function of parathyroid cells in vitro and in hyperparathyroidism. Biochim Biophys Acta. 1988 Feb 22;968(2):253–260. doi: 10.1016/0167-4889(88)90014-6. [DOI] [PubMed] [Google Scholar]
- Nygren P., Larsson R. Differential in vitro sensitivity of human tumor and normal cells to chemotherapeutic agents and resistance modulators. Int J Cancer. 1991 Jun 19;48(4):598–604. doi: 10.1002/ijc.2910480419. [DOI] [PubMed] [Google Scholar]
- Nygren P., Larsson R. Modulation of vincristine sensitivity of human kidney tumor cells by pharmacological agents interfering with intracellular signals. No apparent relationship to changes in cytoplasmic Ca2+ or pH. Biochim Biophys Acta. 1990 May 22;1052(3):392–398. doi: 10.1016/0167-4889(90)90148-7. [DOI] [PubMed] [Google Scholar]
- Nygren P., Larsson R. Verapamil and cyclosporin A sensitize human kidney tumor cells to vincristine in absence of membrane P-glycoprotein and without apparent changes in the cytoplasmic free Ca2+ concentration. Biosci Rep. 1990 Apr;10(2):231–237. doi: 10.1007/BF01116583. [DOI] [PubMed] [Google Scholar]
- Oakes S. G., Schlager J. J., Santone K. S., Abraham R. T., Powis G. Doxorubicin blocks the increase in intracellular Ca++, part of a second messenger system in N1E-115 murine neuroblastoma cells. J Pharmacol Exp Ther. 1990 Mar;252(3):979–983. [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Reeve J. G., Rabbitts P. H., Twentyman P. R. Non-P-glycoprotein-mediated multidrug resistance with reduced EGF receptor expression in a human large cell lung cancer cell line. Br J Cancer. 1990 Jun;61(6):851–855. doi: 10.1038/bjc.1990.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safa A. R., Glover C. J., Sewell J. L., Meyers M. B., Biedler J. L., Felsted R. L. Identification of the multidrug resistance-related membrane glycoprotein as an acceptor for calcium channel blockers. J Biol Chem. 1987 Jun 5;262(16):7884–7888. [PubMed] [Google Scholar]
- Scheper R. J., Bulte J. W., Brakkee J. G., Quak J. J., van der Schoot E., Balm A. J., Meijer C. J., Broxterman H. J., Kuiper C. M., Lankelma J. Monoclonal antibody JSB-1 detects a highly conserved epitope on the P-glycoprotein associated with multi-drug-resistance. Int J Cancer. 1988 Sep 15;42(3):389–394. doi: 10.1002/ijc.2910420314. [DOI] [PubMed] [Google Scholar]
- Slater L. M., Sweet P., Stupecky M., Gupta S. Cyclosporin A reverses vincristine and daunorubicin resistance in acute lymphatic leukemia in vitro. J Clin Invest. 1986 Apr;77(4):1405–1408. doi: 10.1172/JCI112450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen A. M., Luthman H., Hellgren D., Lambert B. Levels of hypoxanthine phosphoribosyltransferase RNA in human cells. Exp Cell Res. 1990 Feb;186(2):236–244. doi: 10.1016/0014-4827(90)90301-p. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Kawabata H., Tsukagoshi S., Sakurai Y. High calcium content of pleiotropic drug-resistant P388 and K562 leukemia and Chinese hamster ovary cells. Cancer Res. 1984 Nov;44(11):5095–5099. [PubMed] [Google Scholar]
- Ueda K., Clark D. P., Chen C. J., Roninson I. B., Gottesman M. M., Pastan I. The human multidrug resistance (mdr1) gene. cDNA cloning and transcription initiation. J Biol Chem. 1987 Jan 15;262(2):505–508. [PubMed] [Google Scholar]
- Vayuvegula B., Slater L., Meador J., Gupta S. Correction of altered plasma membrane potentials. A possible mechanism of cyclosporin A and verapamil reversal of pleiotropic drug resistance in neoplasia. Cancer Chemother Pharmacol. 1988;22(2):163–168. doi: 10.1007/BF00257315. [DOI] [PubMed] [Google Scholar]
- Yusa K., Tsuruo T. Reversal mechanism of multidrug resistance by verapamil: direct binding of verapamil to P-glycoprotein on specific sites and transport of verapamil outward across the plasma membrane of K562/ADM cells. Cancer Res. 1989 Sep 15;49(18):5002–5006. [PubMed] [Google Scholar]


