Abstract
The effects of N-[2-(methylamino)ethyl]-5-isoquinolinesulfonamide (H-8) and 1-(5-isoquinolinesulfonyl)-2-methylpiperazine (H-7) on the growth of P388 and its multidrug-resistant (MDR) variants were examined with the objective of assessing the possible changes in cyclic nucleotide-dependent protein kinases and protein kinase C-mediated pathways associated with MDR. H-8, an inhibitor of cyclic nucleotide-dependent protein kinases, inhibited the growth of the parental P388 murine leukaemic cells, but not that of MDR variants up to 200 microM. However the growth of both drug-sensitive and resistant cell lines were uniformly inhibited by H-7. Both the cytotoxic and cytokinetic results revealed that the growth-inhibition by H-8 of P388 cells is mainly due to a blockade of cell-cycle progression rather than due to a killing of cells. The degree of resistance to H-8 was directly proportional to their extent of resistance to vincristine, adriamycin, and 4'-demethylepipodophyllotoxin-9-(4,6-O-ethylidene)-beta-D-gluco pyr anoside (VP-16) and to that of the expression of P-glycoprotein. These findings raised the possibility that P-glycoprotein might play a role in the cross-resistance to H-8. To test the hypothesis, we examined the effect of H-8 on the binding of 3H-vincristine to membrane fraction isolated from P388/VCR-600 cells and on the enhancement of cytotoxicity to anticancer drugs in MDR cells. H-8 did not have any influences on these reactions. Thus, the cross-resistance to H-8 may be mediated through a mechanism different from an overexpression of P-glycoprotein.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

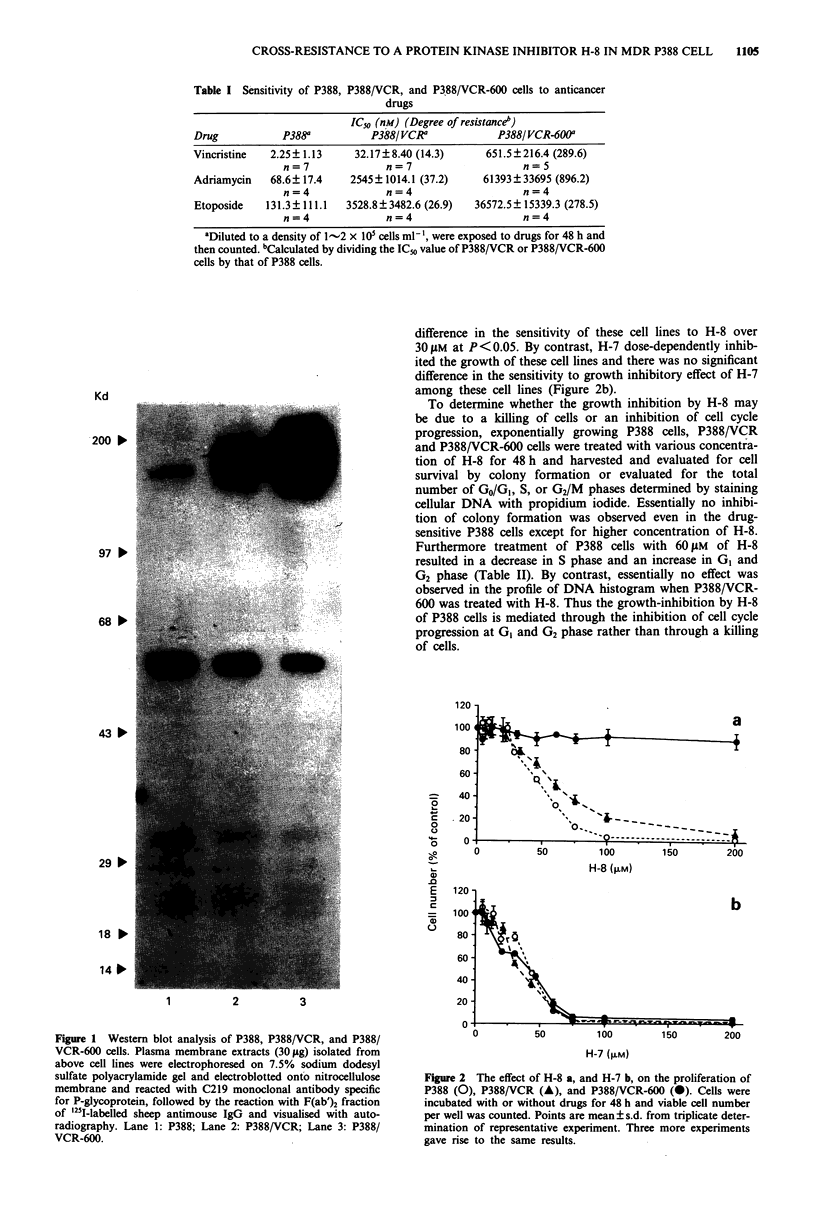
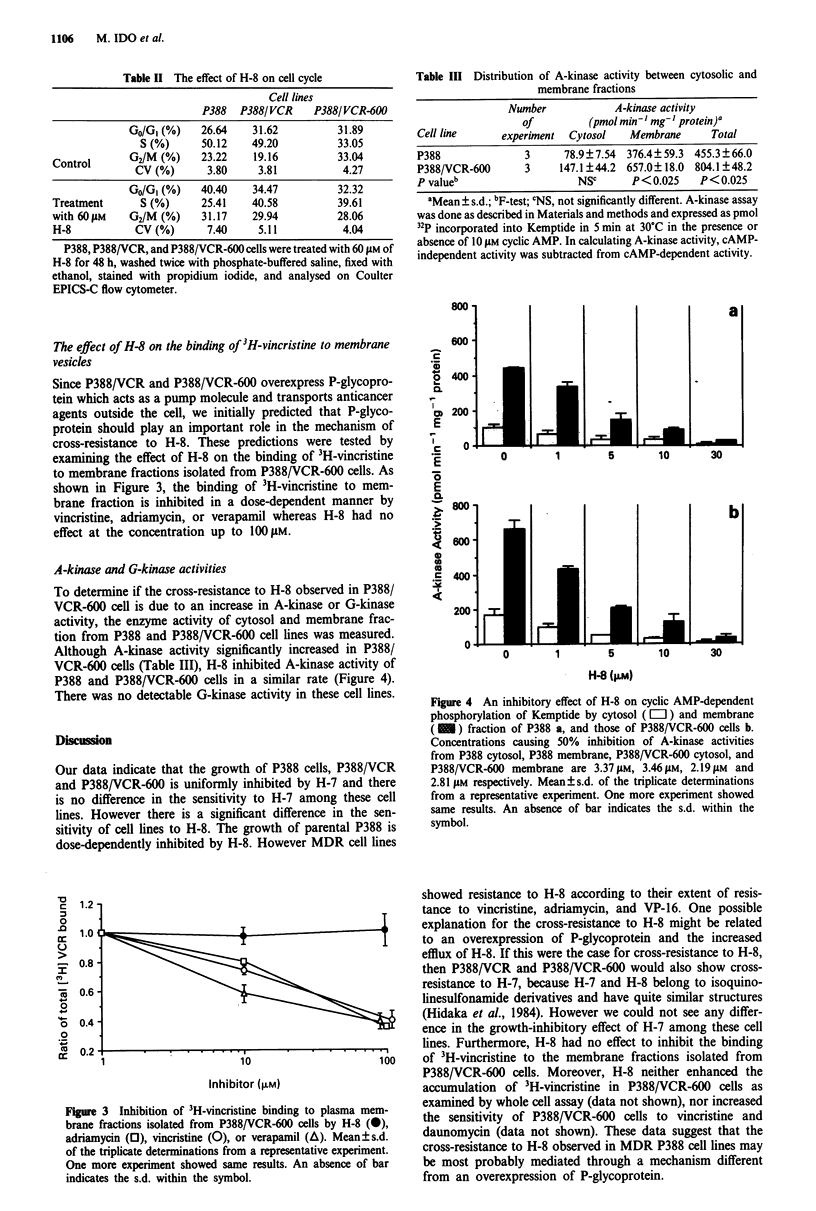

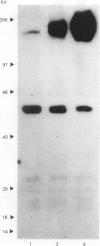
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Beck W. T., Mueller T. J., Tanzer L. R. Altered surface membrane glycoproteins in Vinca alkaloid-resistant human leukemic lymphoblasts. Cancer Res. 1979 Jun;39(6 Pt 1):2070–2076. [PubMed] [Google Scholar]
- Biedler J. L., Riehm H., Peterson R. H., Spengler B. A. Membrane-mediated drug resistance and phenotypic reversion to normal growth behavior of Chinese hamster cells. J Natl Cancer Inst. 1975 Sep;55(3):671–680. doi: 10.1093/jnci/55.3.671. [DOI] [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Cornwell M. M., Safa A. R., Felsted R. L., Gottesman M. M., Pastan I. Membrane vesicles from multidrug-resistant human cancer cells contain a specific 150- to 170-kDa protein detected by photoaffinity labeling. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3847–3850. doi: 10.1073/pnas.83.11.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffie A. M., Alam T., Seneviratne C., Beenken S. W., Batra J. K., Shea T. C., Henner W. D., Goldenberg G. J. Multifactorial resistance to adriamycin: relationship of DNA repair, glutathione transferase activity, drug efflux, and P-glycoprotein in cloned cell lines of adriamycin-sensitive and -resistant P388 leukemia. Cancer Res. 1988 Jul 1;48(13):3595–3602. [PubMed] [Google Scholar]
- Gerlach J. H., Endicott J. A., Juranka P. F., Henderson G., Sarangi F., Deuchars K. L., Ling V. Homology between P-glycoprotein and a bacterial haemolysin transport protein suggests a model for multidrug resistance. Nature. 1986 Dec 4;324(6096):485–489. doi: 10.1038/324485a0. [DOI] [PubMed] [Google Scholar]
- Gros P., Croop J., Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986 Nov 7;47(3):371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- Hamada H., Tsuruo T. Purification of the 170- to 180-kilodalton membrane glycoprotein associated with multidrug resistance. 170- to 180-kilodalton membrane glycoprotein is an ATPase. J Biol Chem. 1988 Jan 25;263(3):1454–1458. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Ido M., Asao T., Sakurai M., Inagaki M., Saito M., Hidaka H. An inhibitor of protein kinase C, 1-(5-isoquinolinylsulfonyl)-2-methylpiperazine(H-7) inhibits TPA-induced reduction of vincristine uptake from P388 murine leukemic cell. Leuk Res. 1986;10(9):1063–1069. doi: 10.1016/0145-2126(86)90050-0. [DOI] [PubMed] [Google Scholar]
- Ido M., Sato K., Sakurai M., Inagaki M., Saitoh M., Watanabe M., Hidaka H. Decreased phorbol ester receptor and protein kinase C in P388 murine leukemic cells resistant to etoposide. Cancer Res. 1987 Jul 1;47(13):3460–3463. [PubMed] [Google Scholar]
- Inaba M., Johnson R. K. Decreased retention of actinomycin D as the basis for cross-resistance in anthracycline-resistant sublines of P388 leukemia. Cancer Res. 1977 Dec;37(12):4629–4634. [PubMed] [Google Scholar]
- Inagaki M., Watanabe M., Hidaka H. N-(2-Aminoethyl)-5-isoquinolinesulfonamide, a newly synthesized protein kinase inhibitor, functions as a ligand in affinity chromatography. Purification of Ca2+-activated, phospholipid-dependent and other protein kinases. J Biol Chem. 1985 Mar 10;260(5):2922–2925. [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maller J. L., Kemp B. E., Krebs E. G. In vivo phosphorylation of a synthetic peptide substrate of cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1978 Jan;75(1):248–251. doi: 10.1073/pnas.75.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers M. B., Merluzzi V. J., Spengler B. A., Biedler J. L. Epidermal growth factor receptor is increased in multidrug-resistant Chinese hamster and mouse tumor cells. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5521–5525. doi: 10.1073/pnas.83.15.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito M., Hamada H., Tsuruo T. ATP/Mg2+-dependent binding of vincristine to the plasma membrane of multidrug-resistant K562 cells. J Biol Chem. 1988 Aug 25;263(24):11887–11891. [PubMed] [Google Scholar]
- Safa A. R., Glover C. J., Meyers M. B., Biedler J. L., Felsted R. L. Vinblastine photoaffinity labeling of a high molecular weight surface membrane glycoprotein specific for multidrug-resistant cells. J Biol Chem. 1986 May 15;261(14):6137–6140. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982 Nov;42(11):4730–4733. [PubMed] [Google Scholar]
- Vickers P. J., Dickson R. B., Shoemaker R., Cowan K. H. A multidrug-resistant MCF-7 human breast cancer cell line which exhibits cross-resistance to antiestrogens and hormone-independent tumor growth in vivo. Mol Endocrinol. 1988 Oct;2(10):886–892. doi: 10.1210/mend-2-10-886. [DOI] [PubMed] [Google Scholar]
- Zuckier G., Tritton T. R. Adriamycin causes up regulation of epidermal growth factor receptors in actively growing cells. Exp Cell Res. 1983 Oct;148(1):155–161. doi: 10.1016/0014-4827(83)90195-7. [DOI] [PubMed] [Google Scholar]