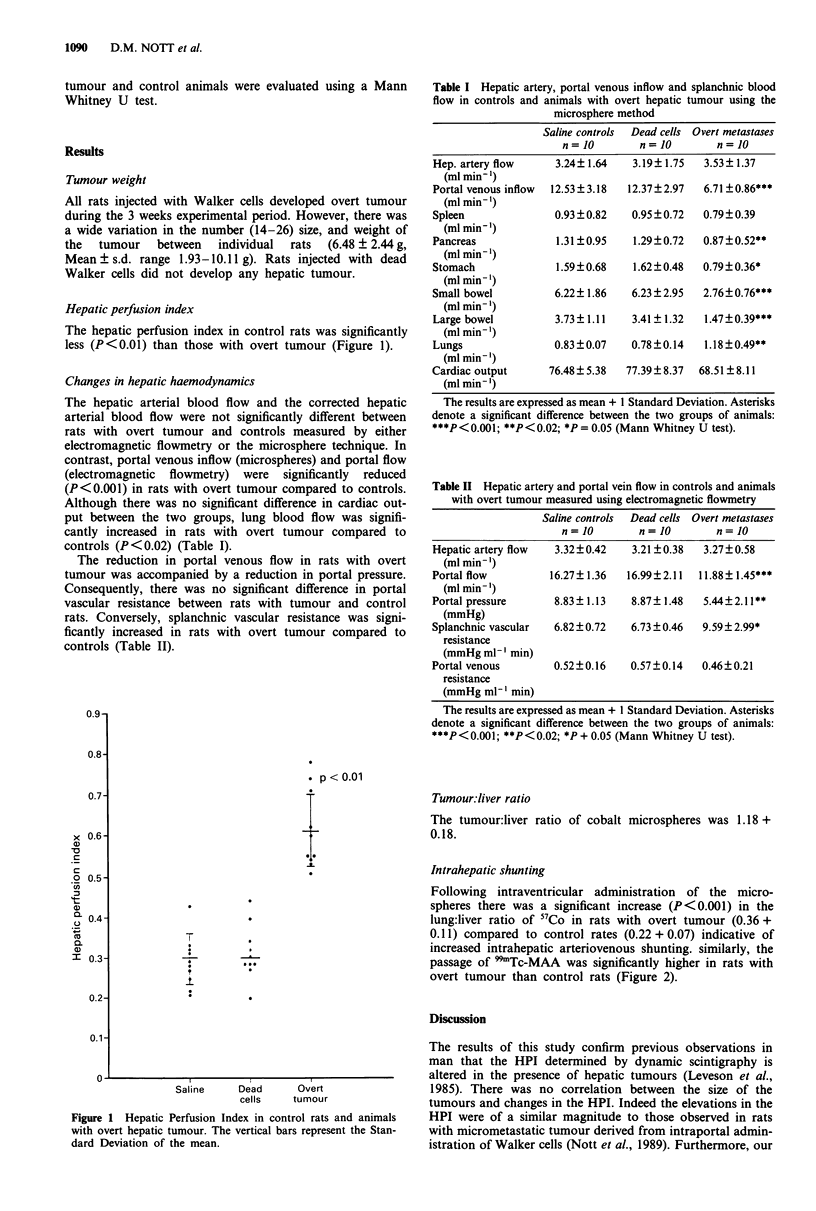
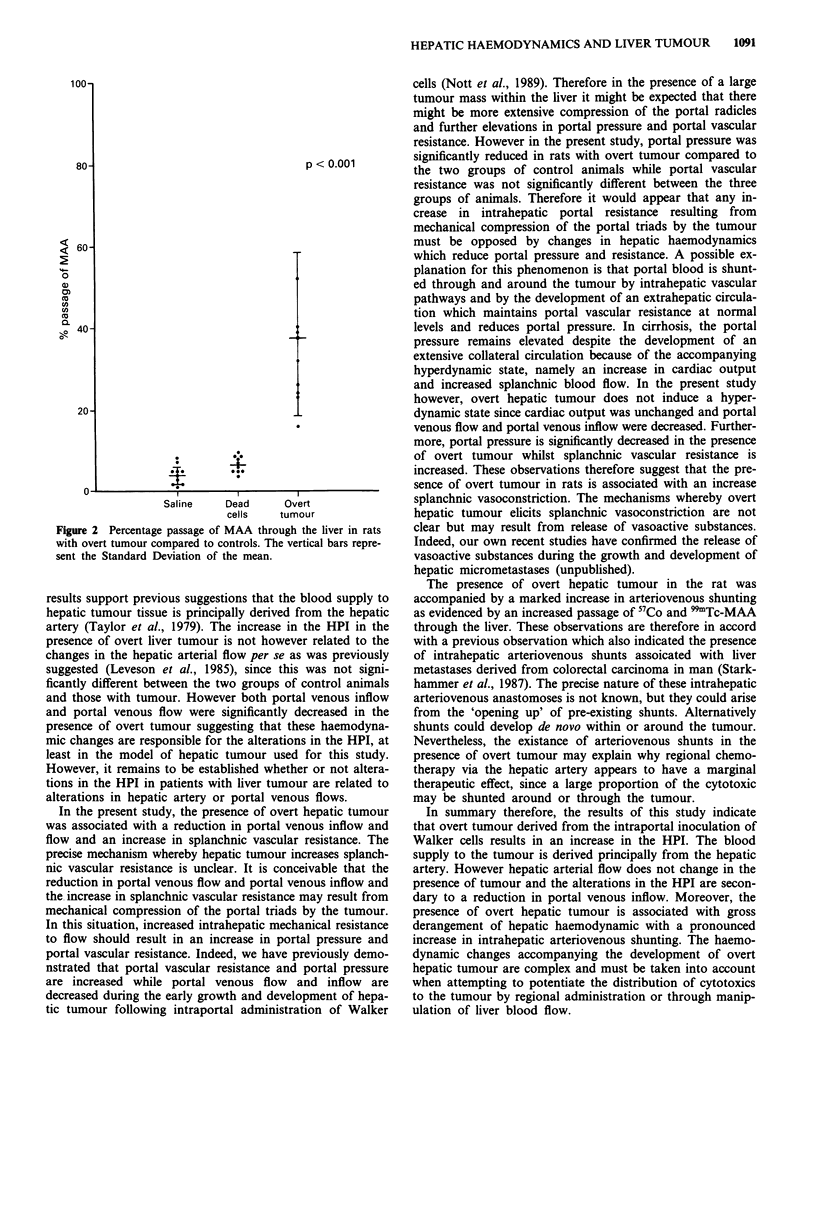
Abstract
Overt liver tumour was induced in Fisher rats by intraportal administration of 1.6 x 10(7) Walker carcinosarcoma cells. Control groups of rats received similar volumes of dead cells or saline intraportally. All animals were studied at 3 weeks when overt tumour was present. The Hepatic Perfusion Index (HPI) was significantly raised in rats with overt tumour compared to both groups of control animals. Portal flow and portal venous inflow were significantly reduced in the presence of overt tumour but hepatic arterial flow did not alter. These observations suggest that the alteration in the HPI in the presence of overt tumour results from an alteration in portal venous flow and inflow even though the blood supply to the tumour is principally derived from the hepatic artery. The changes in hepatic haemodynamics in the presence of tumour were accompanied by a reduction in portal pressure, an increase in splanchnic vascular resistance and an increase in the degree of arteriovenous shunting through the liver. Portal vascular resistance was unchanged. These findings indicate that the presence of overt hepatic tumour results in gross derangements of hepatic blood flow. These changes must be taken into consideration when attempting to potentiate the delivery of cytotoxic drugs to hepatic tumour by manipulation of hepatic haemodynamics.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Leveson S. H., Wiggins P. A., Giles G. R., Parkin A., Robinson P. J. Deranged liver blood flow patterns in the detection of liver metastases. Br J Surg. 1985 Feb;72(2):128–130. doi: 10.1002/bjs.1800720220. [DOI] [PubMed] [Google Scholar]
- Leveson S. H., Wiggins P. A., Nasiru T. A., Giles G. R., Robinson P. J., Parkin A. Improving the detection of hepatic metastases by the use of dynamic flow scintigraphy. Br J Cancer. 1983 May;47(5):719–721. doi: 10.1038/bjc.1983.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt D. G., Nies A. S. Simultaneous measurement of cardiac output and its distribution with microspheres in the rat. Cardiovasc Res. 1976 Jul;10(4):494–498. doi: 10.1093/cvr/10.4.494. [DOI] [PubMed] [Google Scholar]
- Nott D. M., Grime S. J., Yates J., Day D. W., Baxter J. N., Jenkins S. A., Cooke T. G. Changes in the hepatic perfusion index during the development of experimental hepatic tumours. Br J Surg. 1989 Mar;76(3):259–263. doi: 10.1002/bjs.1800760315. [DOI] [PubMed] [Google Scholar]
- Starkhammar H., Håkansson L., Morales O., Svedberg J. Effect of microspheres in intra-arterial chemotherapy. A study of arterio-venous shunting and passage of a labelled marker. Med Oncol Tumor Pharmacother. 1987;4(2):87–96. doi: 10.1007/BF02934945. [DOI] [PubMed] [Google Scholar]


