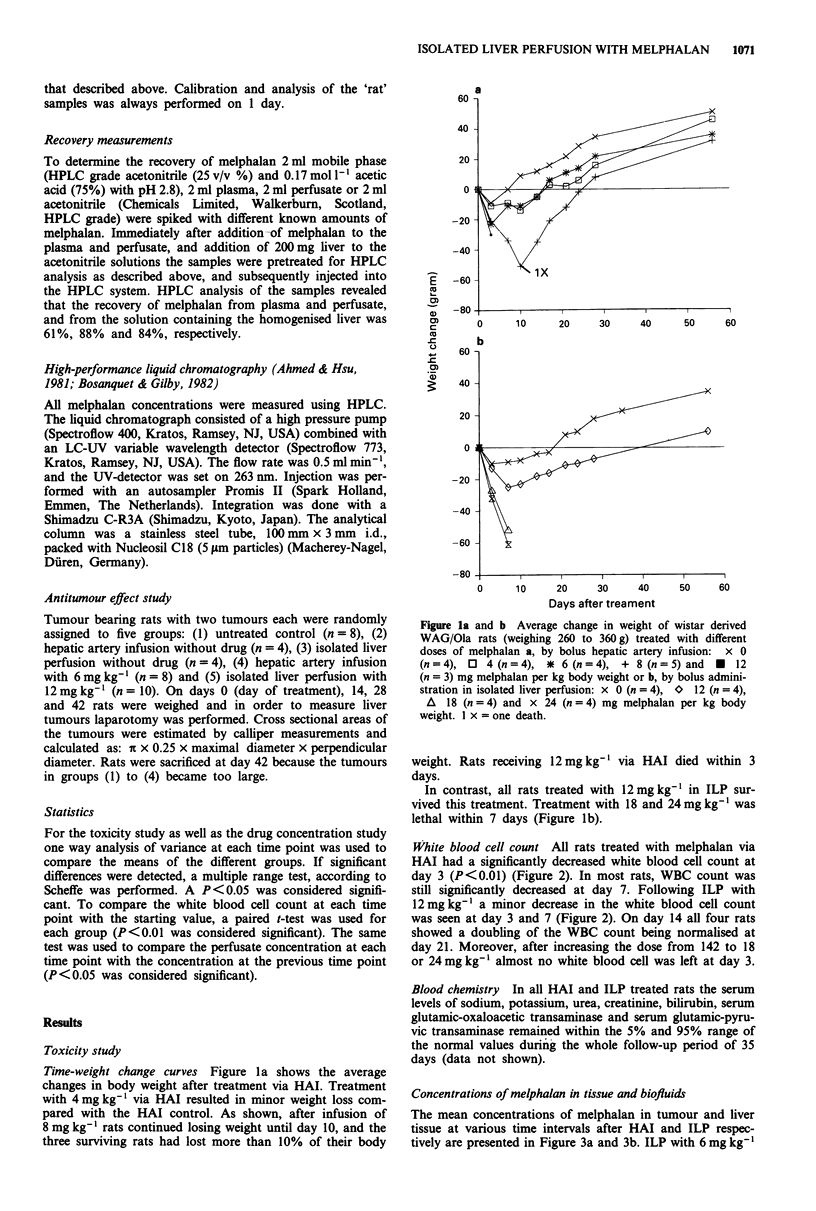
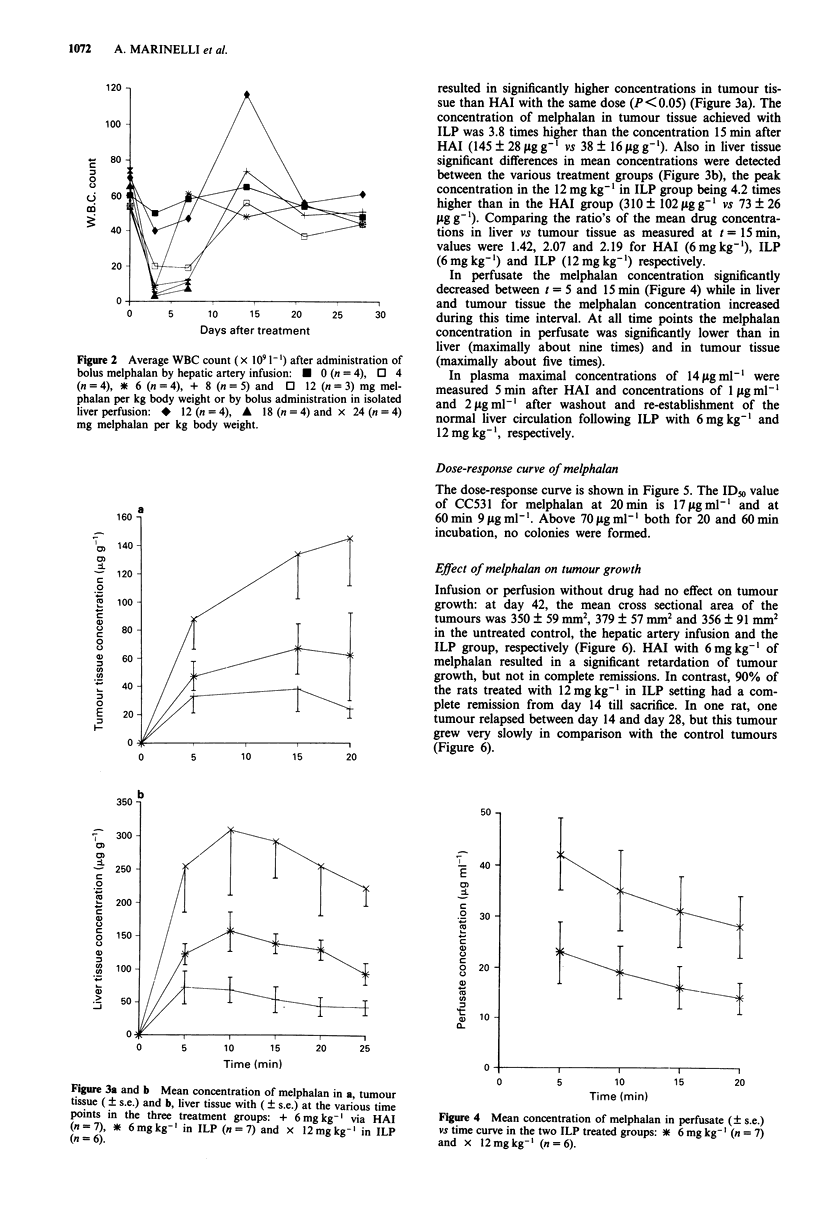
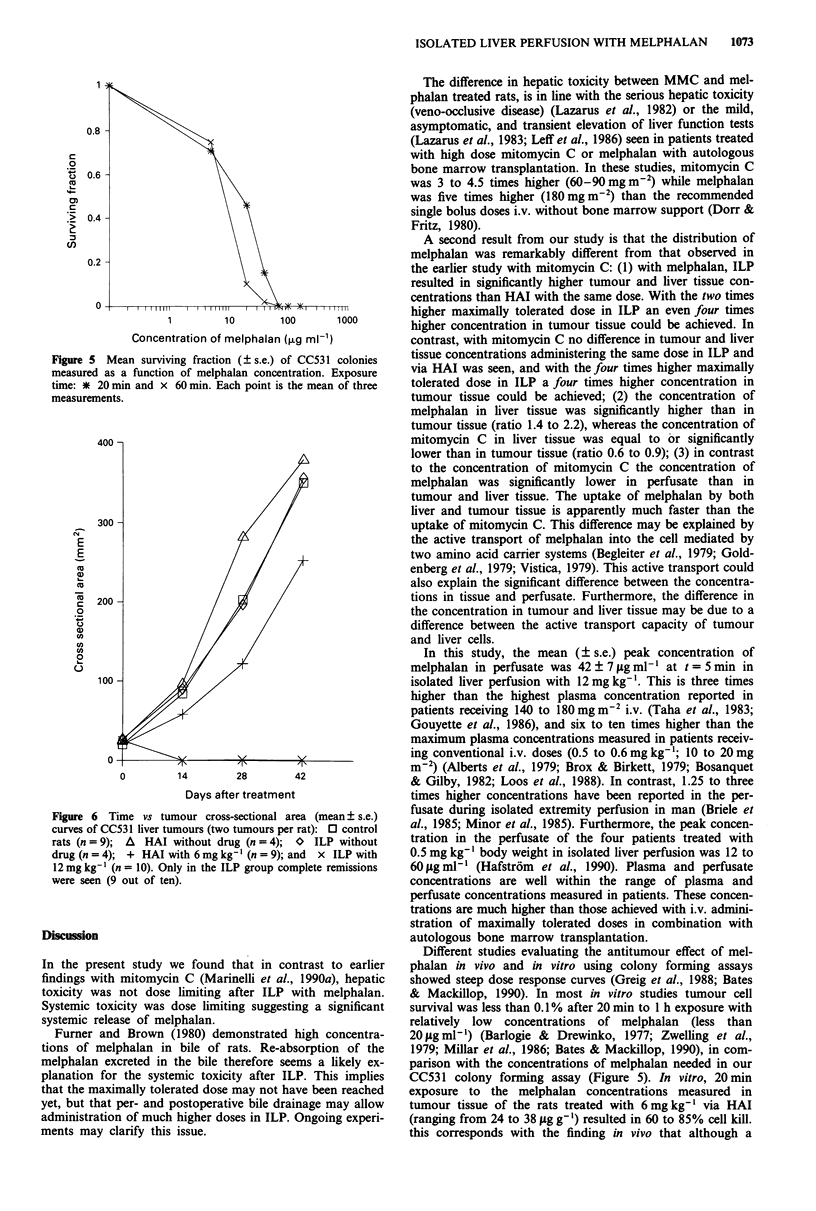
Abstract
Regional chemotherapy allows further exploitation of the steep dose response curve of most chemotherapeutic agents, while systemic toxicity remains tolerable. We investigated the difference in maximally tolerated dose, pharmacokinetics and antitumour effect comparing administration of melphalan as a bolus in isolated liver perfusion (ILP) or via hepatic artery infusion (HAI). For these in vivo studies an experimental model for liver metastases in male WAG/Ola rats is obtained by subcapsular inoculation of CC531 rat colon carcinoma cells. In this system, ILP allowed administration of a two times higher dose than HAI (12 mg kg-1 vs 6 mg kg-1). In both treatment modalities systemic toxicity (leukopenia) was dose limiting. No hepatic toxicity was observed. Bolus administration of the maximally tolerated doses of melphalan in HAI (6 mg kg-1) and ILP (12 mg kg-1) resulted in four times higher concentrations in both liver and tumour tissue of the ILP treated rats. However, the ratio of mean drug concentration in liver vs tumour tissue appeared to be 1.5 times that found for HAI. In the range of the in tumour tissue measured melphalan concentrations the CC531 cells showed a steep dose response relationship in vitro. Whereas HAI resulted in significant tumour growth delay, complete remissions were observed in 90% of the rats treated with ILP. This study shows that with 12 mg kg-1 melphalan in ILP highly effective drug concentrations are achieved in CC531 tumour tissue; although the melphalan concentration in liver tissue shows an even higher increase than in tumour tissue, hepatic toxicity is negligible in this dose range.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. E., Hsu T. F. Quantitative analysis of melphalan and its major hydrolysate in patients and animals by reversed-phase high-performance liquid chromatography. J Chromatogr. 1981 Mar 13;222(3):453–460. doi: 10.1016/s0378-4347(00)84146-8. [DOI] [PubMed] [Google Scholar]
- Aigner K., Walther H., Tonn J. C., Krahl M., Wenzl A., Merker G., Schwemmle K. Die isolierte Leberperfusion mit 5-Fluorouracil (5-FU) beim Menschen. Chirurg. 1982 Sep;53(9):571–573. [PubMed] [Google Scholar]
- August D. A., Ottow R. T., Sugarbaker P. H. Clinical perspective of human colorectal cancer metastasis. Cancer Metastasis Rev. 1984;3(4):303–324. doi: 10.1007/BF00051457. [DOI] [PubMed] [Google Scholar]
- Barlogie B., Drewinko B. Lethal and kinetic response of cultured human lymphoid cells to melphalan. Cancer Treat Rep. 1977 May-Jun;61(3):425–436. [PubMed] [Google Scholar]
- Bates D. A., Mackillop W. J. The effect of hyperthermia in combination with melphalan on drug-sensitive and drug-resistant CHO cells in vitro. Br J Cancer. 1990 Aug;62(2):183–188. doi: 10.1038/bjc.1990.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begleiter A., Lam H. Y., Grover J., Froese E., Goldenberg G. J. Evidence for active transport of melphalan by two amino acid carriers in L5178Y lymphoblasts in vitro. Cancer Res. 1979 Feb;39(2 Pt 1):353–359. [PubMed] [Google Scholar]
- Bosanquet A. G., Gilby E. D. Measurement of plasma melphalan at therapeutic concentrations using isocratic high-performance liquid chromatography. J Chromatogr. 1982 Nov 12;232(2):345–354. doi: 10.1016/s0378-4347(00)84174-2. [DOI] [PubMed] [Google Scholar]
- Briele H. A., Djuric M., Jung D. T., Mortell T., Patel M. K., Das Gupta T. K. Pharmacokinetics of melphalan in clinical isolation perfusion of the extremities. Cancer Res. 1985 Apr;45(4):1885–1889. [PubMed] [Google Scholar]
- Brox L., Birkett L., Belch A. Pharmacology of intravenous melphalan in patients with multiple myeloma. Cancer Treat Rev. 1979 Jun;6 (Suppl):27–32. doi: 10.1016/s0305-7372(79)80007-9. [DOI] [PubMed] [Google Scholar]
- Cady B. Natural history of primary and secondary tumors of the liver. Semin Oncol. 1983 Jun;10(2):127–134. [PubMed] [Google Scholar]
- Chang A. E., Schneider P. D., Sugarbaker P. H., Simpson C., Culnane M., Steinberg S. M. A prospective randomized trial of regional versus systemic continuous 5-fluorodeoxyuridine chemotherapy in the treatment of colorectal liver metastases. Ann Surg. 1987 Dec;206(6):685–693. doi: 10.1097/00000658-198712000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furner R. L., Brown R. K. L-phenylalanine mustard (L-PAM): the first 25 years. Cancer Treat Rep. 1980 Apr-May;64(4-5):559–574. [PubMed] [Google Scholar]
- Goldenberg G. J., Lam H. Y., Begleiter A. Active carrier-mediated transport of melphalan by two separate amino acid transport systems in LPC-1 plasmacytoma cells in vitro. J Biol Chem. 1979 Feb 25;254(4):1057–1064. [PubMed] [Google Scholar]
- Gouyette A., Hartmann O., Pico J. L. Pharmacokinetics of high-dose melphalan in children and adults. Cancer Chemother Pharmacol. 1986;16(2):184–189. doi: 10.1007/BF00256174. [DOI] [PubMed] [Google Scholar]
- Greig N. H., Sweeney D. J., Rapoport S. I. Comparative brain and plasma pharmacokinetics and anticancer activities of chlorambucil and melphalan in the rat. Cancer Chemother Pharmacol. 1988;21(1):1–8. doi: 10.1007/BF00262729. [DOI] [PubMed] [Google Scholar]
- Hohn D. C., Stagg R. J., Friedman M. A., Hannigan J. F., Jr, Rayner A., Ignoffo R. J., Acord P., Lewis B. J. A randomized trial of continuous intravenous versus hepatic intraarterial floxuridine in patients with colorectal cancer metastatic to the liver: the Northern California Oncology Group trial. J Clin Oncol. 1989 Nov;7(11):1646–1654. doi: 10.1200/JCO.1989.7.11.1646. [DOI] [PubMed] [Google Scholar]
- Hughes K. S., Simon R., Songhorabodi S., Adson M. A., Ilstrup D. M., Fortner J. G., Maclean B. J., Foster J. H., Daly J. M., Fitzherbert D. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986 Aug;100(2):278–284. [PubMed] [Google Scholar]
- Kemeny N., Daly J., Reichman B., Geller N., Botet J., Oderman P. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma. A randomized trial. Ann Intern Med. 1987 Oct;107(4):459–465. doi: 10.7326/0003-4819-107-4-459. [DOI] [PubMed] [Google Scholar]
- Kemeny N., Niedzwiecki D., Shurgot B., Oderman P. Prognostic variables in patients with hepatic metastases from colorectal cancer. Importance of medical assessment of liver involvement. Cancer. 1989 Feb 15;63(4):742–747. doi: 10.1002/1097-0142(19890215)63:4<742::aid-cncr2820630423>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Lazarus H. M., Gottfried M. R., Herzig R. H., Phillips G. L., Weiner R. S., Sarna G. P., Fay J., Wolff S. N., Sudilovsky O., Gale R. P. Veno-occlusive disease of the liver after high-dose mitomycin C therapy and autologous bone marrow transplantation. Cancer. 1982 May 1;49(9):1789–1795. doi: 10.1002/1097-0142(19820501)49:9<1789::aid-cncr2820490910>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Lazarus H. M., Herzig R. H., Graham-Pole J., Wolff S. N., Phillips G. L., Strandjord S., Hurd D., Forman W., Gordon E. M., Coccia P. Intensive melphalan chemotherapy and cryopreserved autologous bone marrow transplantation for the treatment of refractory cancer. J Clin Oncol. 1983 Jun;1(6):359–367. doi: 10.1200/JCO.1983.1.6.359. [DOI] [PubMed] [Google Scholar]
- Leff R. S., Thompson J. M., Johnson D. B., Mosley K. R., Daly M. B., Knight W. A., 3rd, Ruxer R. L., Jr, Messerschmidt G. L. Phase II trial of high-dose melphalan and autologous bone marrow transplantation for metastatic colon carcinoma. J Clin Oncol. 1986 Nov;4(11):1586–1591. doi: 10.1200/JCO.1986.4.11.1586. [DOI] [PubMed] [Google Scholar]
- Loos U., Musch E., Engel M., Hartlapp J. H., Hügl E., Dengler H. J. The pharmacokinetics of melphalan during intermittent therapy of multiple myeloma. Eur J Clin Pharmacol. 1988;35(2):187–193. doi: 10.1007/BF00609251. [DOI] [PubMed] [Google Scholar]
- Marinelli A., Dijkstra F. R., van Dierendonck J. H., Kuppen P. J., Cornelisse C. J., van de Velde C. J. Effectiveness of isolated liver perfusion with mitomycin C in the treatment of liver tumours of rat colorectal cancer. Br J Cancer. 1991 Jul;64(1):74–78. doi: 10.1038/bjc.1991.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquet R. L., Westbroek D. L., Jeekel J. Interferon treatment of a transplantable rat colon adenocarcinoma: importance of tumor site. Int J Cancer. 1984 May 15;33(5):689–692. doi: 10.1002/ijc.2910330521. [DOI] [PubMed] [Google Scholar]
- Martin J. K., Jr, O'Connell M. J., Wieand H. S., Fitzgibbons R. J., Jr, Mailliard J. A., Rubin J., Nagorney D. M., Tschetter L. K., Krook J. E. Intra-arterial floxuridine vs systemic fluorouracil for hepatic metastases from colorectal cancer. A randomized trial. Arch Surg. 1990 Aug;125(8):1022–1027. doi: 10.1001/archsurg.1990.01410200086013. [DOI] [PubMed] [Google Scholar]
- Millar B. C., Tilby M. J., Ormerod M. G., Payne A. W., Jinks S., Loverock P. S. Comparative studies of total cross-linking, cell survival and cell cycle perturbations in Chinese hamster cells treated with alkylating agents in vitro. Biochem Pharmacol. 1986 Apr 1;35(7):1163–1169. doi: 10.1016/0006-2952(86)90155-3. [DOI] [PubMed] [Google Scholar]
- Minor D. R., Allen R. E., Alberts D., Peng Y. M., Tardelli G., Hutchinson J. A clinical and pharmacokinetic study of isolated limb perfusion with heat and melphalan for melanoma. Cancer. 1985 Jun 1;55(11):2638–2644. doi: 10.1002/1097-0142(19850601)55:11<2638::aid-cncr2820551118>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Radnell M., Jeppsson B., Bengmark S. A technique for isolated liver perfusion in the rat with survival and results of cytotoxic drug perfusion on liver tumor growth. J Surg Res. 1990 Nov;49(5):394–399. doi: 10.1016/0022-4804(90)90186-6. [DOI] [PubMed] [Google Scholar]
- Silverberg E., Lubera J. A. Cancer statistics, 1988. CA Cancer J Clin. 1988 Jan-Feb;38(1):5–22. doi: 10.3322/canjclin.38.1.5. [DOI] [PubMed] [Google Scholar]
- Skibba J. L., Almagro U. A., Condon R. E., Petroff R. J., Jr A technique for isolation perfusion of the canine liver with survival. J Surg Res. 1983 Feb;34(2):123–132. doi: 10.1016/0022-4804(83)90051-3. [DOI] [PubMed] [Google Scholar]
- Taha I. A., Ahmad R. A., Rogers D. W., Pritchard J., Rogers H. J. Pharmacokinetics of melphalan in children following high-dose intravenous injection. Cancer Chemother Pharmacol. 1983;10(3):212–216. doi: 10.1007/BF00255766. [DOI] [PubMed] [Google Scholar]
- Vistica D. T. Cellular pharmacokinetics of the phenylalanine mustards. Pharmacol Ther. 1983;22(3):379–406. doi: 10.1016/0163-7258(83)90009-8. [DOI] [PubMed] [Google Scholar]
- Vistica D. T. Cytotoxicity as an indicator for transport mechanism: evidence that melphalan is transported by two leucine-preferring carrier systems in the L1210 murine leukemia cell. Biochim Biophys Acta. 1979 Jan 19;550(2):309–317. doi: 10.1016/0005-2736(79)90217-7. [DOI] [PubMed] [Google Scholar]
- Wagner J. S., Adson M. A., Van Heerden J. A., Adson M. H., Ilstrup D. M. The natural history of hepatic metastases from colorectal cancer. A comparison with resective treatment. Ann Surg. 1984 May;199(5):502–508. doi: 10.1097/00000658-198405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood C. B., Gillis C. R., Blumgart L. H. A retrospective study of the natural history of patients with liver metastases from colorectal cancer. Clin Oncol. 1976 Sep;2(3):285–288. [PubMed] [Google Scholar]
- Zwelling L. A., Filipski J., Kohn K. W. Effect of thiourea on survival and DNA cross-link formation in cells treated with platinum(II) complexes, L-phenylalanine mustard, and bis(2-chloroethyl)methylamine. Cancer Res. 1979 Dec;39(12):4989–4995. [PubMed] [Google Scholar]
- de Brauw L. M., van de Velde C. J., Tjaden U. R., de Bruijn E. A., Bell A. V., Hermans J., Zwaveling A. In vivo isolated liver perfusion technique in a rat hepatic metastasis model: 5-fluorouracil concentrations in tumor tissue. J Surg Res. 1988 Feb;44(2):137–145. doi: 10.1016/0022-4804(88)90041-8. [DOI] [PubMed] [Google Scholar]
- van de Velde C. J., Kothuis B. J., Barenbrug H. W., Jongejan N., Runia R. D., de Brauw L. M., Zwaveling A. A successful technique of in vivo isolated liver perfusion in pigs. J Surg Res. 1986 Dec;41(6):593–599. doi: 10.1016/0022-4804(86)90084-3. [DOI] [PubMed] [Google Scholar]


