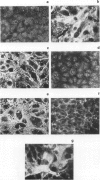
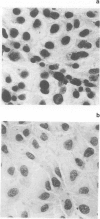
Abstract
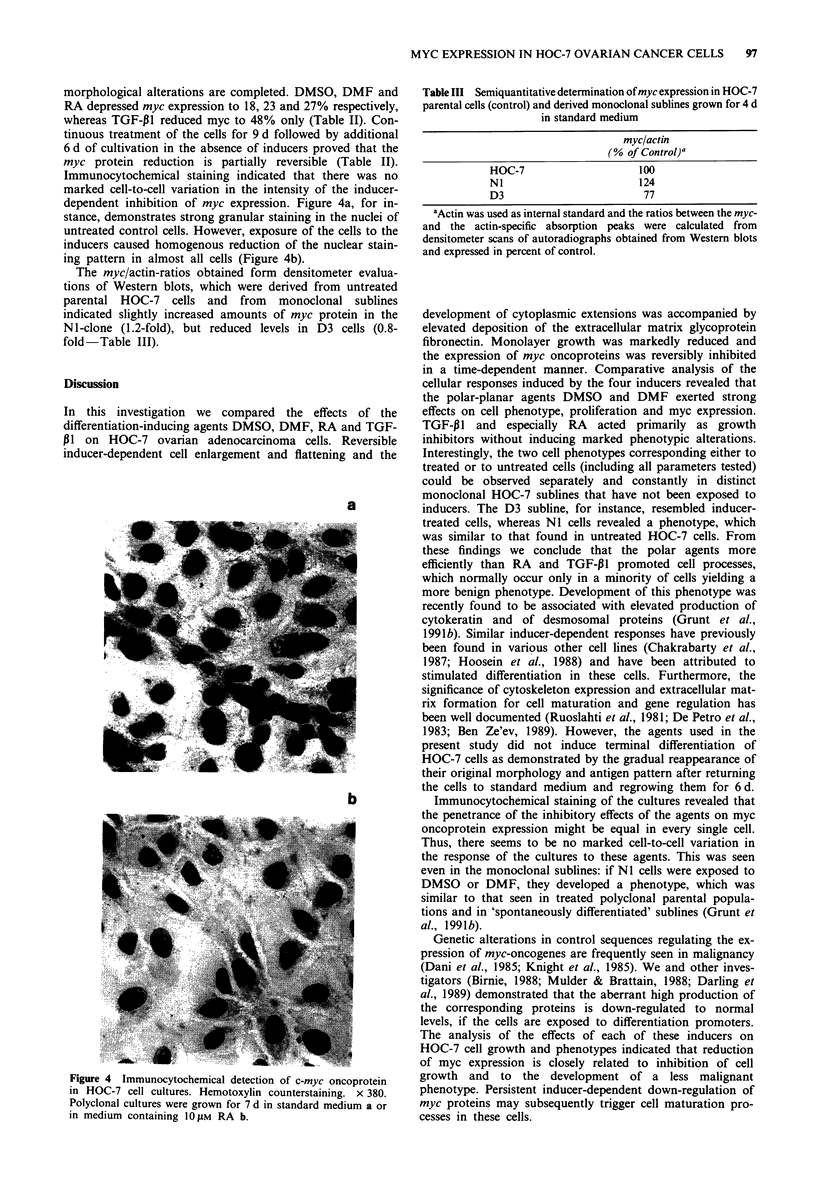
In this investigation we demonstrate expression of myc oncoproteins in HOC-7 ovarian adenocarcinoma cells. The cells were exposed to differentiation inducing agents such as dimethyl sulfoxide (DMSO), N,N-dimethylformamide (DMF), retinoic acid (RA) and transforming growth factor-beta 1 (TGF-beta 1). Myc protein expression in treated cells was then compared with that in control cultures and in monoclonal HOC-7 sublines, which are characterised by distinct phenotypes. Cells exposed to DMSO and DMF became markedly enlarged and flattened and developed cytoplasmic extensions. They looked similar to a subline, which revealed a less malignant and more differentiated cell phenotype. All four inducers prolonged the cell doubling time and reduced the saturation density to levels, normally found in the more differentiated subline. Furthermore, all inducers except RA elevated extracellular fibronectin, which is characteristic for less malignant epithelial cell phenotypes. All four agents inhibited myc oncoprotein expression reversibly (1% DMSO greater than 0.5% DMF greater than 10 microM RA greater than 10 ng ml-1 TGF-beta 1) and in time-dependent manner. Down-regulation of myc protein expression is, therefore, closely related to inducer-dependent growth reduction of HOC-7 cells and to the development of a less malignant cell phenotype.
Full text
PDF

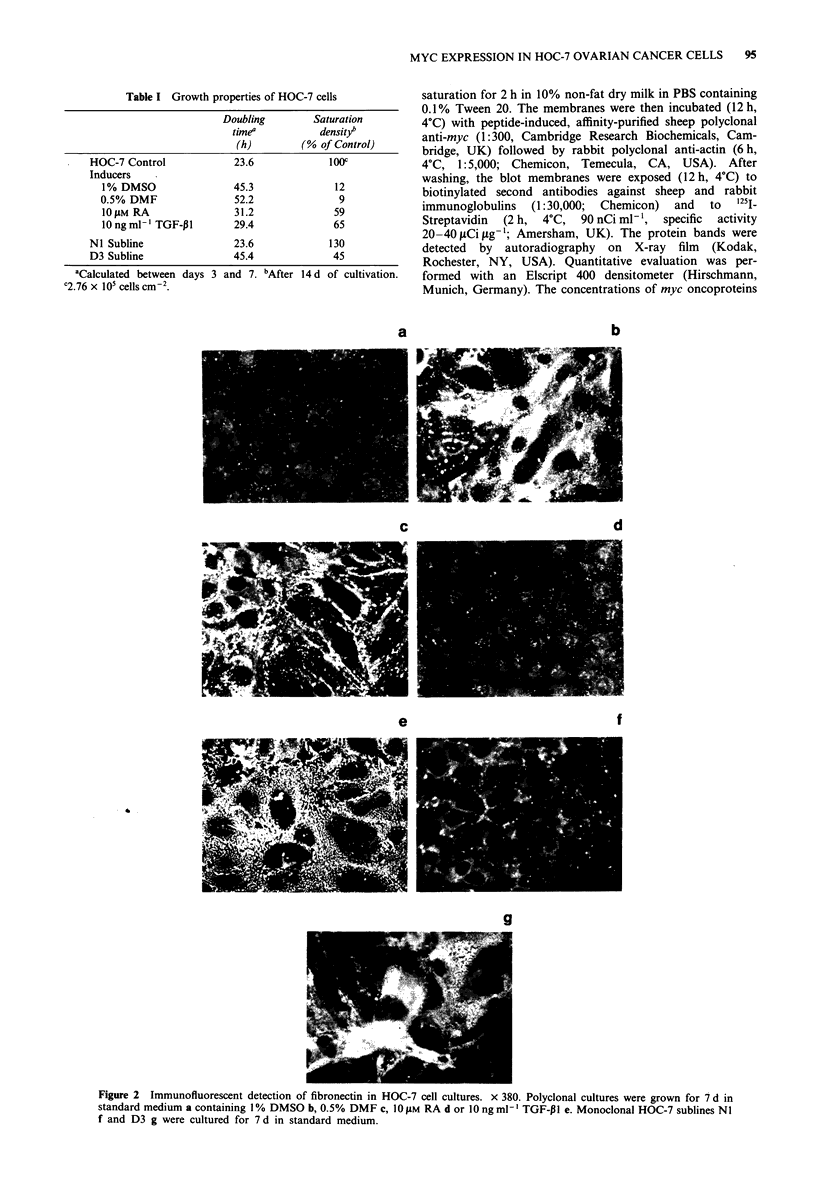
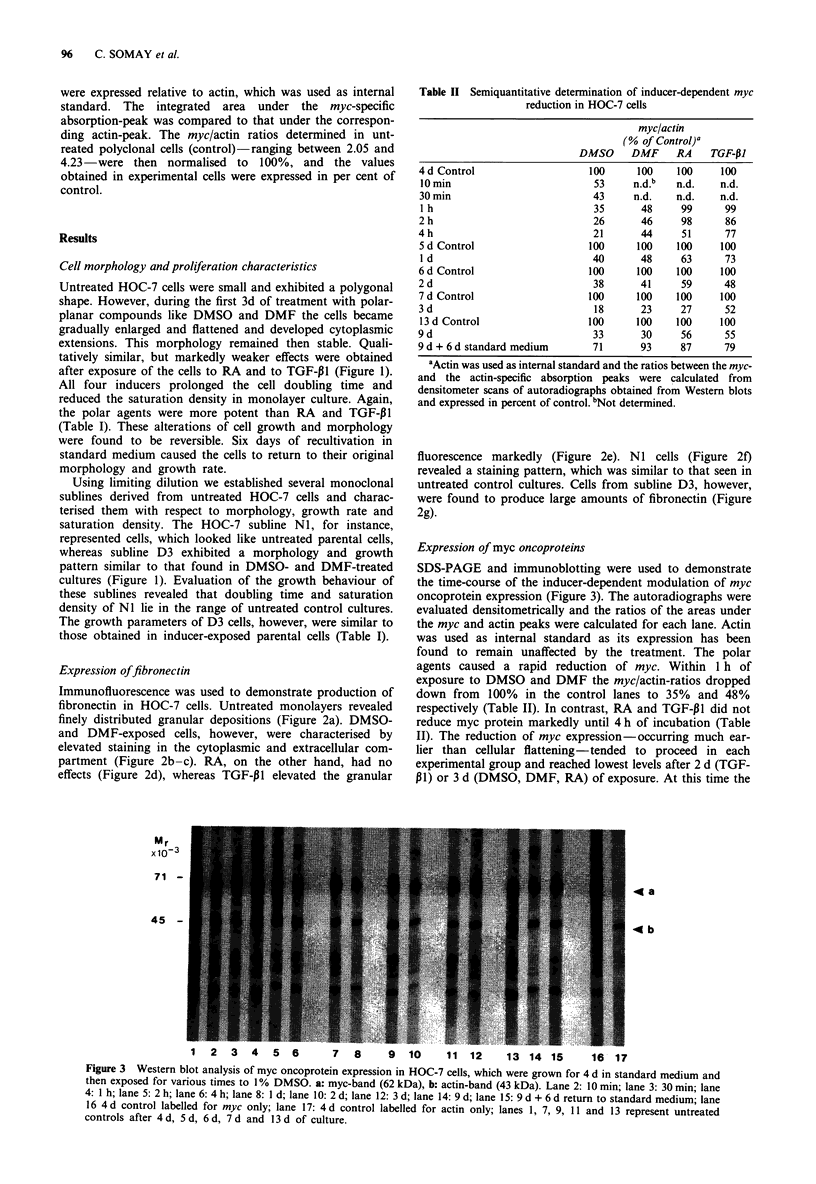

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Ramsay G., Bishop J. M., Pfeifer S. O., Colby W. W., Levinson A. D. Identification of nuclear proteins encoded by viral and cellular myc oncogenes. Nature. 1983 Nov 17;306(5940):274–277. doi: 10.1038/306274a0. [DOI] [PubMed] [Google Scholar]
- Buick R. N., Pullano R., Trent J. M. Comparative properties of five human ovarian adenocarcinoma cell lines. Cancer Res. 1985 Aug;45(8):3668–3676. [PubMed] [Google Scholar]
- Chakrabarty S., Brattain M. G., Ochs R. L., Varani J. Modulation of fibronectin, laminin, and cellular adhesion in the transformation and differentiation of murine AKR fibroblasts. J Cell Physiol. 1987 Dec;133(3):415–425. doi: 10.1002/jcp.1041330302. [DOI] [PubMed] [Google Scholar]
- Dani C., Mechti N., Piechaczyk M., Lebleu B., Jeanteur P., Blanchard J. M. Increased rate of degradation of c-myc mRNA in interferon-treated Daudi cells. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4896–4899. doi: 10.1073/pnas.82.15.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling D., Tavassoli M., Linskens M. H., Farzaneh F. DMSO induced modulation of c-myc steady-state RNA levels in a variety of different cell lines. Oncogene. 1989 Feb;4(2):175–179. [PubMed] [Google Scholar]
- De Petro G., Barlati S., Vartio T., Vaheri A. Transformation-enhancing activity in plasma of tumor patients: relationship with fibronectin fragments. Int J Cancer. 1983 Feb 15;31(2):157–162. doi: 10.1002/ijc.2910310205. [DOI] [PubMed] [Google Scholar]
- Fukumoto M., Estensen R. D., Sha L., Oakley G. J., Twiggs L. B., Adcock L. L., Carson L. F., Roninson I. B. Association of Ki-ras with amplified DNA sequences, detected in human ovarian carcinomas by a modified in-gel renaturation assay. Cancer Res. 1989 Apr 1;49(7):1693–1697. [PubMed] [Google Scholar]
- Grunt T. W., Somay C., Pavelka M., Ellinger A., Dittrich E., Dittrich C. The effects of dimethyl sulfoxide and retinoic acid on the cell growth and the phenotype of ovarian cancer cells. J Cell Sci. 1991 Nov;100(Pt 3):657–666. doi: 10.1242/jcs.100.3.657. [DOI] [PubMed] [Google Scholar]
- Hoosein N. M., Brattain D. E., McKnight M. K., Childress K. E., Chakrabarty S., Brattain M. G. Comparison of the antiproliferative effects of transforming growth factor-beta, N,N-dimethylformamide and retinoic acid on a human colon carcinoma cell line. Cancer Lett. 1988 Jun 15;40(2):219–232. doi: 10.1016/0304-3835(88)90014-6. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr, Anton E. D., Fahey D., Friedland B. K., Jonak G. J. Interferon regulates c-myc gene expression in Daudi cells at the post-transcriptional level. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1151–1154. doi: 10.1073/pnas.82.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Locker A. P., Dowle C. S., Ellis I. O., Elston C. W., Blamey R. W., Sikora K., Evan G., Robins R. A. c-myc oncogene product expression and prognosis in operable breast cancer. Br J Cancer. 1989 Nov;60(5):669–672. doi: 10.1038/bjc.1989.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. The TGF-beta family of growth and differentiation factors. Cell. 1987 May 22;49(4):437–438. doi: 10.1016/0092-8674(87)90443-0. [DOI] [PubMed] [Google Scholar]
- Mulder K. M., Brattain M. G. Alterations in c-myc expression in relation to maturational status of human colon carcinoma cells. Int J Cancer. 1988 Jul 15;42(1):64–70. doi: 10.1002/ijc.2910420113. [DOI] [PubMed] [Google Scholar]
- Nau M. M., Brooks B. J., Battey J., Sausville E., Gazdar A. F., Kirsch I. R., McBride O. W., Bertness V., Hollis G. F., Minna J. D. L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature. 1985 Nov 7;318(6041):69–73. doi: 10.1038/318069a0. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Reboulleau C. P., Shapiro H. S. Chemical inducers of differentiation cause conformational changes in the chromatin and deoxyribonucleic acid of murine erythroleukemia cells. Biochemistry. 1983 Sep 13;22(19):4512–4517. doi: 10.1021/bi00288a025. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E., Hayman E. G. Fibronectin: current concepts of its structure and functions. Coll Relat Res. 1981;1(1):95–128. doi: 10.1016/s0174-173x(80)80011-2. [DOI] [PubMed] [Google Scholar]
- Sasano H., Garrett C. T., Wilkinson D. S., Silverberg S., Comerford J., Hyde J. Protooncogene amplification and tumor ploidy in human ovarian neoplasms. Hum Pathol. 1990 Apr;21(4):382–391. doi: 10.1016/0046-8177(90)90199-f. [DOI] [PubMed] [Google Scholar]
- Siracký J., Blasko M., Borovanský J. Stimulation of differentiation in human melanoma cells by dimethyl sulphoxide (DMSO). Neoplasma. 1985;32(6):685–688. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Role of retinoids in differentiation and carcinogenesis. Cancer Res. 1983 Jul;43(7):3034–3040. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D. J., Gonzalez-Cadavid N., Ahuja H., Battifora H., Moore G. E., Cline M. J. A unique pattern of proto-oncogene abnormalities in ovarian adenocarcinomas. Cancer. 1988 Oct 15;62(8):1573–1576. doi: 10.1002/1097-0142(19881015)62:8<1573::aid-cncr2820620819>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]