Abstract
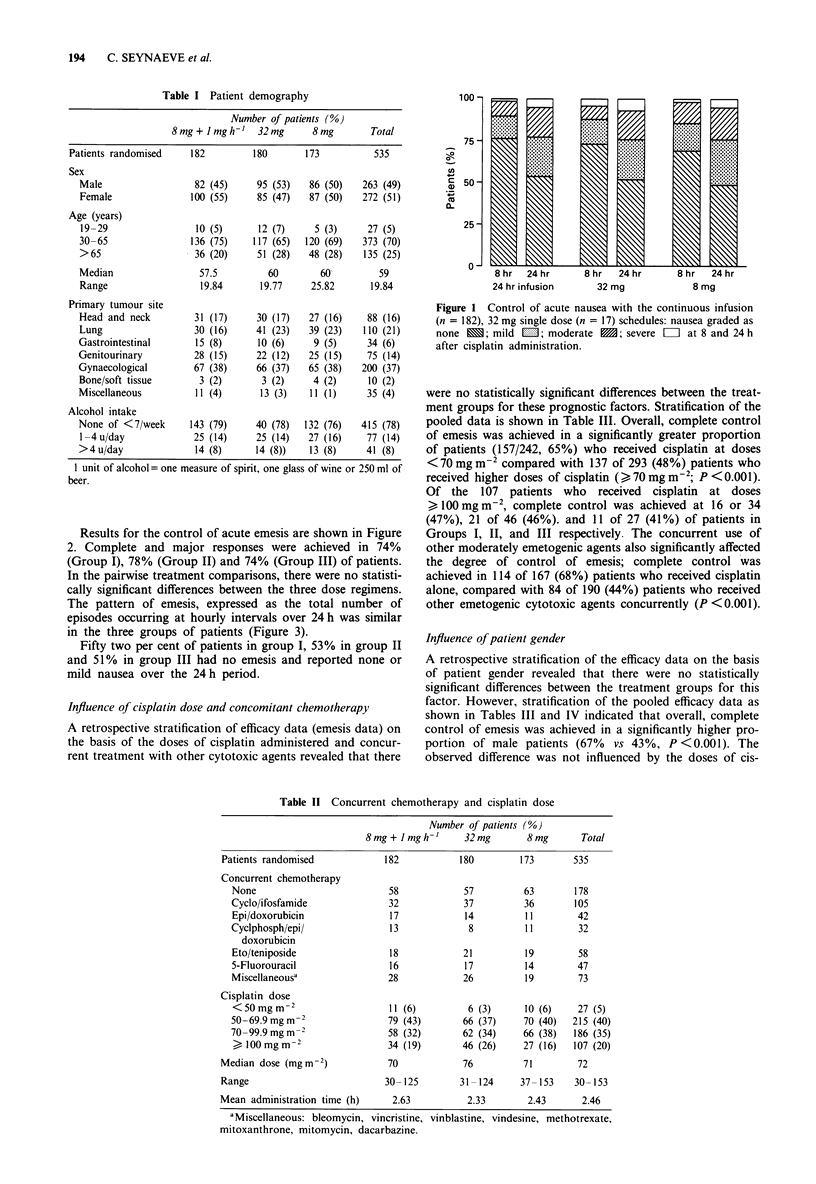
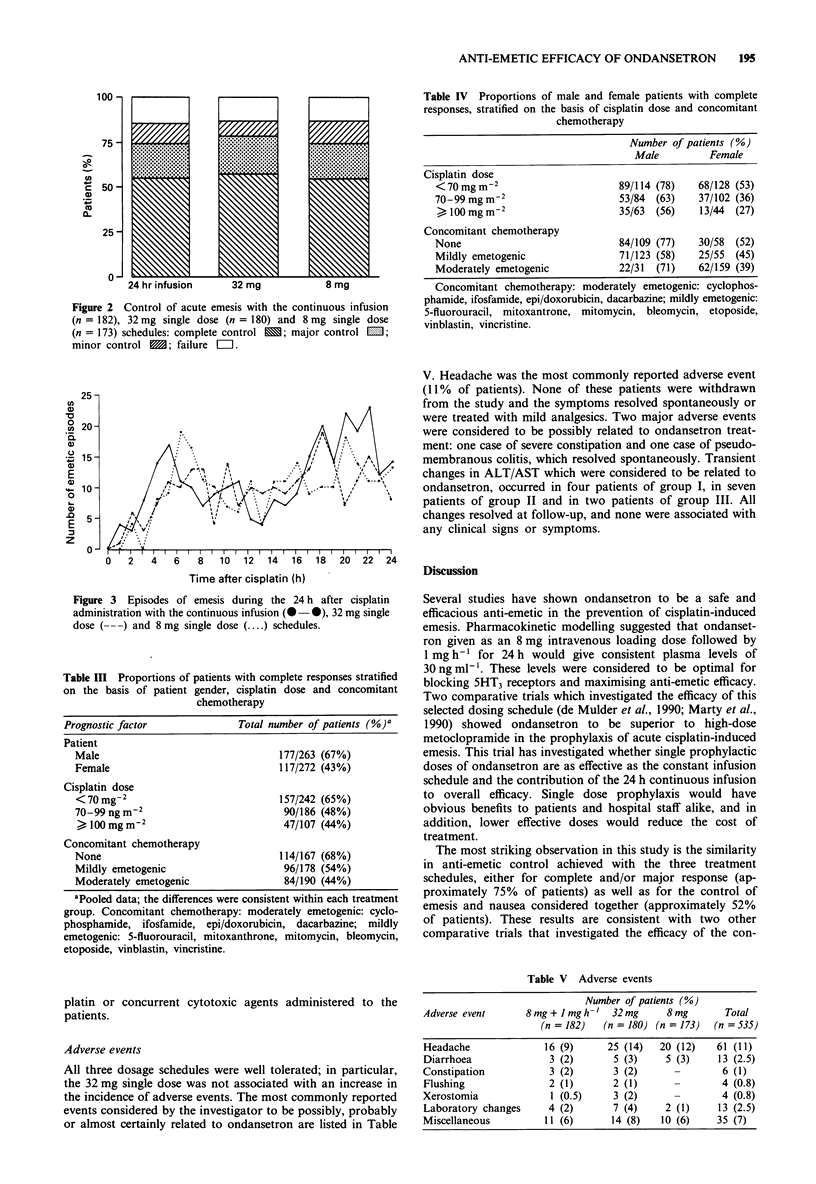
A total of 535 chemotherapy naive, hospitalised patients (263 male/272 female) scheduled to receive cisplatin (50-120 mg m-2)-containing regimens participated in a randomised, double-blind, parallel group study to evaluate the efficacy and safety of three intravenous dose schedules of ondansetron in the prophylaxis of acute nausea and emesis. One hundred and eighty two patients received a loading dose of 8 mg of ondansetron followed by a 24 h infusion of 1 mg h-1 (group 1); 180 and 173 patients received single doses of 32 mg (group II) and 8 mg (group III) respectively, followed by a 24 h placebo infusion. Complete and major control (less than or equal to 2 emetic episodes) of acute emesis was achieved in 74% of patients in group I, 78% in group II and 74% in group III. Seventy seven per cent of the patients in group I, and 75% of patients in groups II and III respectively experienced no or mild nausea during the 24 h observation period. A retrospective stratification of the efficacy data on the basis of patient gender showed the response rate in females to be significant lower (43% vs 67%; less than 0.001). Ondanestron was well tolerated; mild headache was the most commonly reported adverse event (11% of patients) with a similar incidence in the three groups of patients. In conclusion, a single intravenous dose of 8 mg of ondansetron given prior to chemotherapy is as effective as a 32 mg daily dose given as either a single dose of a continuous infusion in the prophylaxis of acute cisplatin-induced emesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carl P. L., Cubeddu L. X., Lindley C., Myers R. D., Rezvani A. H. Do humoral factors mediate cancer chemotherapy-induced emesis? Drug Metab Rev. 1989;21(2):319–333. doi: 10.3109/03602538909029944. [DOI] [PubMed] [Google Scholar]
- Colthup P. V., Palmer J. L. The determination in plasma and pharmacokinetics of ondansetron. Eur J Cancer Clin Oncol. 1989;25 (Suppl 1):S71–S74. [PubMed] [Google Scholar]
- Cubeddu L. X., Hoffmann I. S., Fuenmayor N. T., Finn A. L. Efficacy of ondansetron (GR 38032F) and the role of serotonin in cisplatin-induced nausea and vomiting. N Engl J Med. 1990 Mar 22;322(12):810–816. doi: 10.1056/NEJM199003223221204. [DOI] [PubMed] [Google Scholar]
- De Mulder P. H., Seynaeve C., Vermorken J. B., van Liessum P. A., Mols-Jevdevic S., Allman E. L., Beranek P., Verweij J. Ondansetron compared with high-dose metoclopramide in prophylaxis of acute and delayed cisplatin-induced nausea and vomiting. A multicenter, randomized, double-blind, crossover study. Ann Intern Med. 1990 Dec 1;113(11):834–840. doi: 10.7326/0003-4819-113-11-834. [DOI] [PubMed] [Google Scholar]
- Gralla R. J., Itri L. M., Pisko S. E., Squillante A. E., Kelsen D. P., Braun D. W., Jr, Bordin L. A., Braun T. J., Young C. W. Antiemetic efficacy of high-dose metoclopramide: randomized trials with placebo and prochlorperazine in patients with chemotherapy-induced nausea and vomiting. N Engl J Med. 1981 Oct 15;305(16):905–909. doi: 10.1056/NEJM198110153051601. [DOI] [PubMed] [Google Scholar]
- Hainsworth J., Harvey W., Pendergrass K., Kasimis B., Oblon D., Monaghan G., Gandara D., Hesketh P., Khojasteh A., Harker G. A single-blind comparison of intravenous ondansetron, a selective serotonin antagonist, with intravenous metoclopramide in the prevention of nausea and vomiting associated with high-dose cisplatin chemotherapy. J Clin Oncol. 1991 May;9(5):721–728. doi: 10.1200/JCO.1991.9.5.721. [DOI] [PubMed] [Google Scholar]
- Kris M. G., Gralla R. J., Clark R. A., Tyson L. B., Groshen S. Antiemetic control and prevention of side effects of anti-cancer therapy with lorazepam or diphenhydramine when used in combination with metoclopramide plus dexamethasone. A double-blind, randomized trial. Cancer. 1987 Dec 1;60(11):2816–2822. doi: 10.1002/1097-0142(19871201)60:11<2816::aid-cncr2820601137>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Lazarus H. M., Bryson J. C., Lemon E., Pritchard J. F., Blumer J. Antiemetic efficacy and pharmacokinetic analyses of the serotonin antagonist ondansetron (GR 38032F) during multiple-day chemotherapy with cisplatin prior to autologous bone marrow transplantation. J Natl Cancer Inst. 1990 Nov 21;82(22):1776–1778. doi: 10.1093/jnci/82.22.1776. [DOI] [PubMed] [Google Scholar]
- Martin M., Diaz-Rubio E. Emesis during past pregnancy: a new prognostic factor in chemotherapy-induced emesis. Ann Oncol. 1990;1(2):152–153. doi: 10.1093/oxfordjournals.annonc.a057695. [DOI] [PubMed] [Google Scholar]
- Marty M., Pouillart P., Scholl S., Droz J. P., Azab M., Brion N., Pujade-Lauraine E., Paule B., Paes D., Bons J. Comparison of the 5-hydroxytryptamine3 (serotonin) antagonist ondansetron (GR 38032F) with high-dose metoclopramide in the control of cisplatin-induced emesis. N Engl J Med. 1990 Mar 22;322(12):816–821. doi: 10.1056/NEJM199003223221205. [DOI] [PubMed] [Google Scholar]
- Ortiz J., Artigas F., Gelpí E. Serotonergic status in human blood. Life Sci. 1988;43(12):983–990. doi: 10.1016/0024-3205(88)90543-7. [DOI] [PubMed] [Google Scholar]
- Roila F., Basurto C., Bracarda S., Sassi M., Lupattelli M., Picciafuoco M., Boschetti E., Tonato M., Del Favero A. Double-blind crossover trial of single vs. divided dose of metoclopramide in a combined regimen for treatment of cisplatin-induced emesis. Eur J Cancer. 1991;27(2):119–121. doi: 10.1016/0277-5379(91)90466-q. [DOI] [PubMed] [Google Scholar]
- Roila F., Tonato M., Basurto C., Picciafuoco M., Bracarda S., Donati D., Malacarne P., Monici L., Di Costanzo F., Patoia L. Protection from nausea and vomiting in cisplatin-treated patients: high-dose metoclopramide combined with methylprednisolone versus metoclopramide combined with dexamethasone and diphenhydramine: a study of the Italian Oncology Group for Clinical Research. J Clin Oncol. 1989 Nov;7(11):1693–1700. doi: 10.1200/JCO.1989.7.11.1693. [DOI] [PubMed] [Google Scholar]
- Roila F., Tonato M., Cognetti F., Cortesi E., Favalli G., Marangolo M., Amadori D., Bella M. A., Gramazio V., Donati D. Prevention of cisplatin-induced emesis: a double-blind multicenter randomized crossover study comparing ondansetron and ondansetron plus dexamethasone. J Clin Oncol. 1991 Apr;9(4):675–678. doi: 10.1200/JCO.1991.9.4.675. [DOI] [PubMed] [Google Scholar]
- Tonato M., Roila F., Del Favero A. Methodology of antiemetic trials: a review. Ann Oncol. 1991 Feb;2(2):107–114. doi: 10.1093/oxfordjournals.annonc.a057871. [DOI] [PubMed] [Google Scholar]


