Abstract
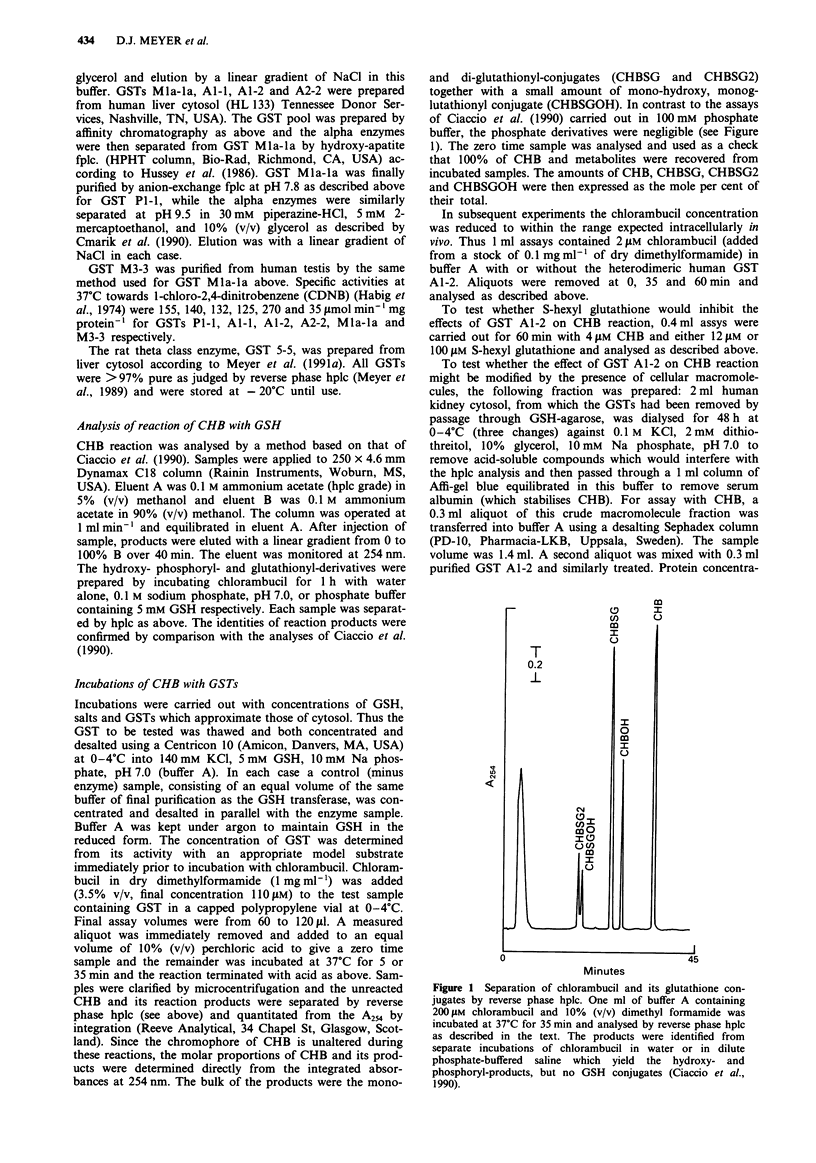
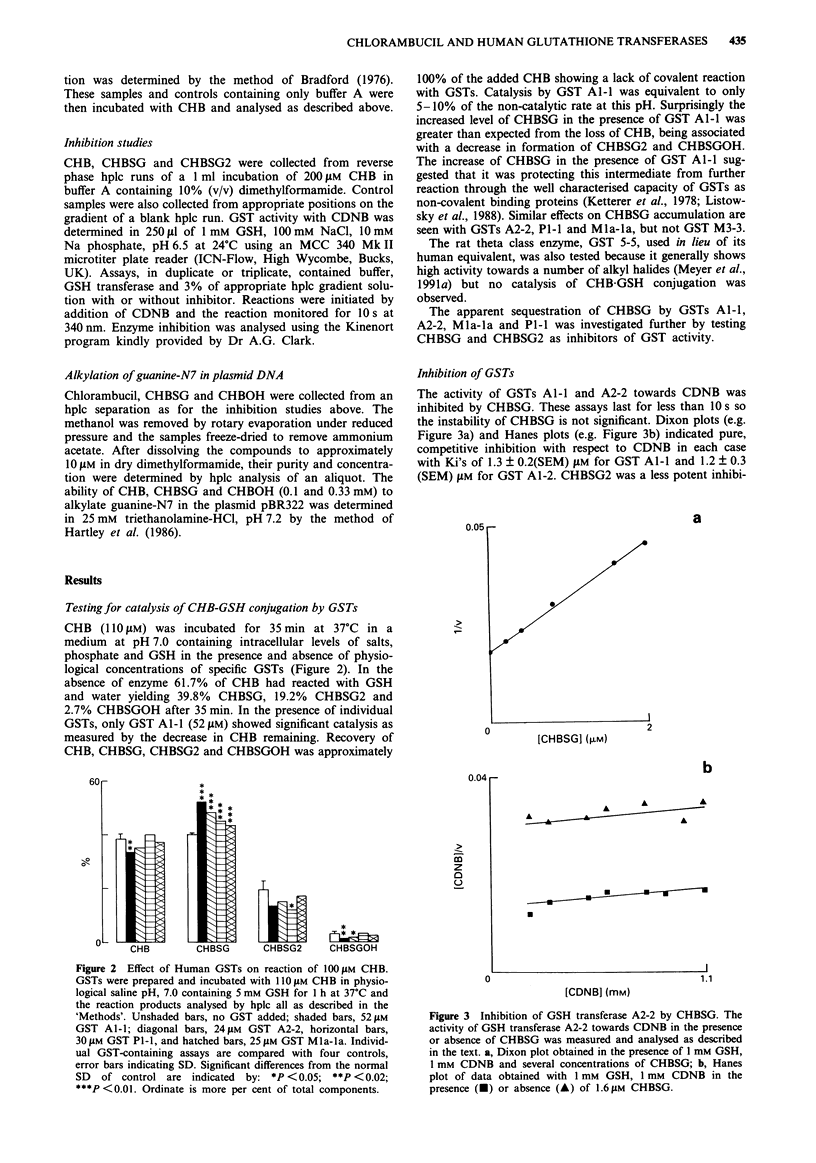
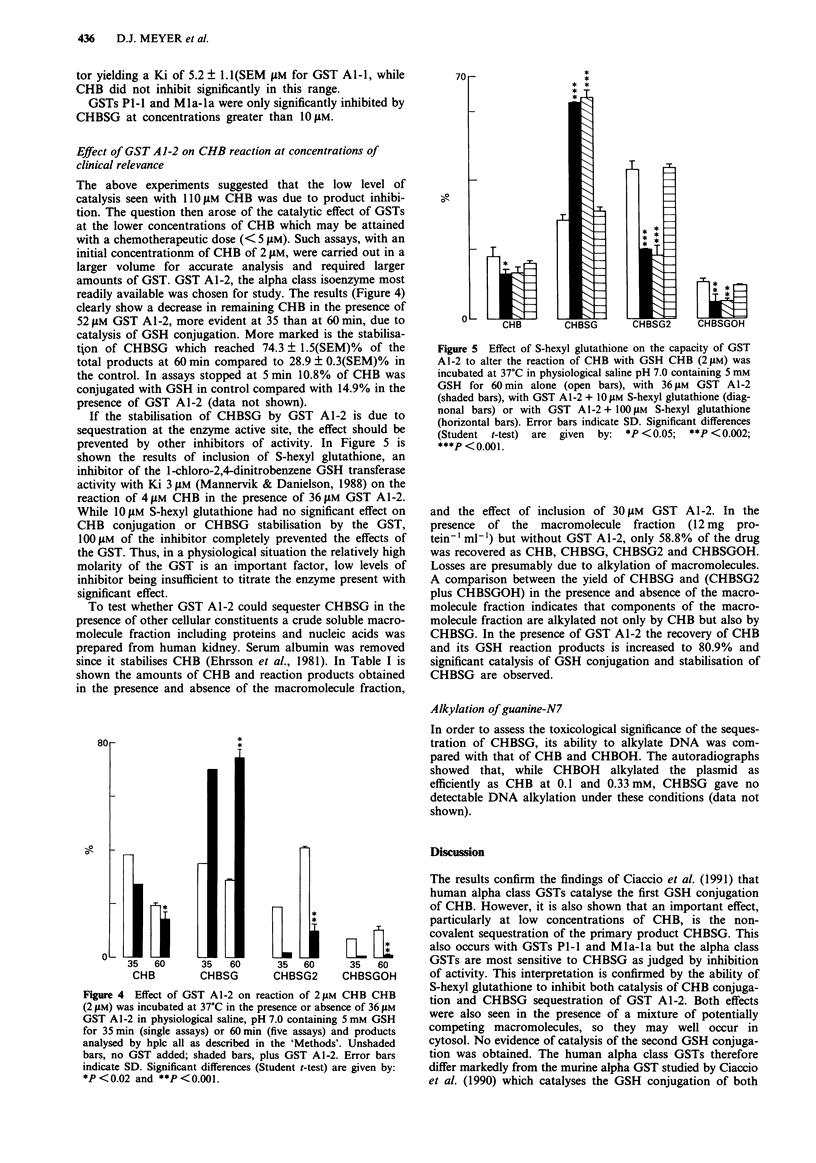
The spontaneous reaction of 110 microM chlorambucil (4-[p-[bis(2-chloroethyl)amino]phenyl]-butanoic acid; CHB) with 5 mM GSH at 37 degrees C in physiological phosphate-buffered saline for 35 min gave primarily the monoglutathionyl derivative, 4-[p-[N-2-chloroethyl,N-2-S-glutathionylethyl]amino]phenyl]-butano ic acid; CHBSG) and the diglutathionyl derivative, 4-[p-[bis(2-S-glutathionylethyl]amino]phenyl]-butanoic acid (CHBSG2) with small amounts of the hydroxy-derivatives: 4-[p-[N-2-chloroethyl,N-2-hydroxy-ethyl]amino] phenyl-butanoic acid (CHBOH) and 4-[p-[N-2-S-glutathionylethyl-2-hydroxyethyl]amino]phenyl]-butanoi c acid (CHBSGOH). The inclusion of approximately physiological amounts of human glutathione S-transferases (GSTs) A1-1, A2-2, P1-1, M1a-1a M3-3 or P1-1 (for nomenclature see Mannervik et al., 1992, Biochem. J., 282, 305) had little or no catalytic effect on these reactions as determined by loss of CHB. However, GTSs A1-1 and A2-2 were associated with a significant increase of CHBSG at the expense of CHBSG2 + CHBSGOH suggesting that these GTs sequestered CHBSG at the active site. This interpretation was supported by inhibition studies which showed that CHBSG was a pure competitive inhibitor of the activity of GSTs A1-1 and A2-2 towards 1-chloro-2,4-dinitrobenzene with Ki's of 1.3 and 1.2 microM respectively. GSH transferases P1-1 and M1a-1a were inhibited by CHBSG above 10 microM. Incubation of 2 microM CHB, a concentration which may be of more significance for chemotherapy, in the presence or absence of GST A1-2 (20-50 microM) showed catalysis of GSH monoconjugation equivalent to 18% of the spontaneous rate. However, the dominant effect again was the sequestration of CHBSG which reached 74.3 +/- 1.5 (SEM)% of the total reactants at 60 min compared to 28.9 +/- 0.3(SEM)% in controls. CHBSG, although possessing a potential electrophilic centre, showed no detectable alkylation of plasmid DNA but indirect evidence was obtained that it alkylated other cellular macromolecules. It is concluded that the contribution of GSTs to catalysis of CHB detoxication will depend on factors not previously considered, namely the relative molarities of CHB, CHBSG and GSTs, and the cellular capacity to excrete CHBSG to relieve product inhibition.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Bartosz G., Sies H. Low- and high-Km transport of dinitrophenyl glutathione in inside out vesicles from human erythrocytes. Biochim Biophys Acta. 1992 Jan 10;1103(1):115–119. doi: 10.1016/0005-2736(92)90064-s. [DOI] [PubMed] [Google Scholar]
- Bank B. B., Kanganis D., Liebes L. F., Silber R. Chlorambucil pharmacokinetics and DNA binding in chronic lymphocytic leukemia lymphocytes. Cancer Res. 1989 Feb 1;49(3):554–559. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buetler T. M., Eaton D. L. Complementary DNA cloning, messenger RNA expression, and induction of alpha-class glutathione S-transferases in mouse tissues. Cancer Res. 1992 Jan 15;52(2):314–318. [PubMed] [Google Scholar]
- Ciaccio P. J., Tew K. D., LaCreta F. P. Enzymatic conjugation of chlorambucil with glutathione by human glutathione S-transferases and inhibition by ethacrynic acid. Biochem Pharmacol. 1991 Sep 12;42(7):1504–1507. doi: 10.1016/0006-2952(91)90468-k. [DOI] [PubMed] [Google Scholar]
- Ciaccio P. J., Tew K. D., LaCreta F. P. The spontaneous and glutathione S-transferase-mediated reaction of chlorambucil with glutathione. Cancer Commun. 1990;2(8):279–285. doi: 10.3727/095535490820874263. [DOI] [PubMed] [Google Scholar]
- Cmarik J. L., Inskeep P. B., Meredith M. J., Meyer D. J., Ketterer B., Guengerich F. P. Selectivity of rat and human glutathione S-transferases in activation of ethylene dibromide by glutathione conjugation and DNA binding and induction of unscheduled DNA synthesis in human hepatocytes. Cancer Res. 1990 May 1;50(9):2747–2752. [PubMed] [Google Scholar]
- Ehrsson H., Lönroth U., Wallin I., Ehrnebo M., Nilsson S. O. Degradation of chlorambucil in aqueous solution--influences of human albumin binding. J Pharm Pharmacol. 1981 May;33(5):313–315. doi: 10.1111/j.2042-7158.1981.tb13787.x. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hartley J. A., Gibson N. W., Kohn K. W., Mattes W. B. DNA sequence selectivity of guanine-N7 alkylation by three antitumor chloroethylating agents. Cancer Res. 1986 Apr;46(4 Pt 2):1943–1947. [PubMed] [Google Scholar]
- Hayes J. D., Judah D. J., McLellan L. I., Kerr L. A., Peacock S. D., Neal G. E. Ethoxyquin-induced resistance to aflatoxin B1 in the rat is associated with the expression of a novel alpha-class glutathione S-transferase subunit, Yc2, which possesses high catalytic activity for aflatoxin B1-8,9-epoxide. Biochem J. 1991 Oct 15;279(Pt 2):385–398. doi: 10.1042/bj2790385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey A. J., Stockman P. K., Beckett G. J., Hayes J. D. Variations in the glutathione S-transferase subunits expressed in human livers. Biochim Biophys Acta. 1986 Nov 7;874(1):1–12. doi: 10.1016/0167-4838(86)90094-4. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Müller M., Klünemann C., Schaub T., Keppler D. ATP-dependent primary active transport of cysteinyl leukotrienes across liver canalicular membrane. Role of the ATP-dependent transport system for glutathione S-conjugates. J Biol Chem. 1990 Nov 5;265(31):19279–19286. [PubMed] [Google Scholar]
- Johnston J. B., Israels L. G., Goldenberg G. J., Anhalt C. D., Verburg L., Mowat M. R., Begleiter A. Glutathione S-transferase activity, sulfhydryl group and glutathione levels, and DNA cross-linking activity with chlorambucil in chronic lymphocytic leukemia. J Natl Cancer Inst. 1990 May 2;82(9):776–779. doi: 10.1093/jnci/82.9.776. [DOI] [PubMed] [Google Scholar]
- Lewis A. D., Hickson I. D., Robson C. N., Harris A. L., Hayes J. D., Griffiths S. A., Manson M. M., Hall A. E., Moss J. E., Wolf C. R. Amplification and increased expression of alpha class glutathione S-transferase-encoding genes associated with resistance to nitrogen mustards. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8511–8515. doi: 10.1073/pnas.85.22.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyland-Jones B. R., Townsend A. J., Tu C. P., Cowan K. H., Goldsmith M. E. Antineoplastic drug sensitivity of human MCF-7 breast cancer cells stably transfected with a human alpha class glutathione S-transferase gene. Cancer Res. 1991 Jan 15;51(2):587–594. [PubMed] [Google Scholar]
- Listowsky I., Abramovitz M., Homma H., Niitsu Y. Intracellular binding and transport of hormones and xenobiotics by glutathione-S-transferases. Drug Metab Rev. 1988;19(3-4):305–318. doi: 10.3109/03602538808994138. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Awasthi Y. C., Board P. G., Hayes J. D., Di Ilio C., Ketterer B., Listowsky I., Morgenstern R., Muramatsu M., Pearson W. R. Nomenclature for human glutathione transferases. Biochem J. 1992 Feb 15;282(Pt 1):305–306. doi: 10.1042/bj2820305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. J., Coles B., Pemble S. E., Gilmore K. S., Fraser G. M., Ketterer B. Theta, a new class of glutathione transferases purified from rat and man. Biochem J. 1991 Mar 1;274(Pt 2):409–414. doi: 10.1042/bj2740409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. J., Gilmore K. S., Coles B., Dalton K., Hulbert P. B., Ketterer B. Structural distinction of rat GSH transferase subunit 10. Biochem J. 1991 Mar 1;274(Pt 2):619–619. doi: 10.1042/bj2740619a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. J., Lalor E., Coles B., Kispert A., Alin P., Mannervik B., Ketterer B. Single-step purification and h.p.l.c. analysis of glutathione transferase 8-8 in rat tissues. Biochem J. 1989 Jun 15;260(3):785–788. doi: 10.1042/bj2600785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa K., Saijo N., Tsuchida S., Sakai M., Tsunokawa Y., Yokota J., Muramatsu M., Sato K., Terada M., Tew K. D. Glutathione-S-transferase pi as a determinant of drug resistance in transfectant cell lines. J Biol Chem. 1990 Mar 15;265(8):4296–4301. [PubMed] [Google Scholar]
- O'Dwyer P. J., LaCreta F., Nash S., Tinsley P. W., Schilder R., Clapper M. L., Tew K. D., Panting L., Litwin S., Comis R. L. Phase I study of thiotepa in combination with the glutathione transferase inhibitor ethacrynic acid. Cancer Res. 1991 Nov 15;51(22):6059–6065. [PubMed] [Google Scholar]
- Oude Elferink R. P., Ottenhoff R., Radominska A., Hofmann A. F., Kuipers F., Jansen P. L. Inhibition of glutathione-conjugate secretion from isolated hepatocytes by dipolar bile acids and other organic anions. Biochem J. 1991 Feb 15;274(Pt 1):281–286. doi: 10.1042/bj2740281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploemen J. H., van Ommen B., van Bladeren P. J. Inhibition of rat and human glutathione S-transferase isoenzymes by ethacrynic acid and its glutathione conjugate. Biochem Pharmacol. 1990 Oct 1;40(7):1631–1635. doi: 10.1016/0006-2952(90)90465-w. [DOI] [PubMed] [Google Scholar]
- Puchalski R. B., Fahl W. E. Expression of recombinant glutathione S-transferase pi, Ya, or Yb1 confers resistance to alkylating agents. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2443–2447. doi: 10.1073/pnas.87.7.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal S. S., Sharma R., Gupta S., Ahmad H., Zimniak P., Radominska A., Lester R., Awasthi Y. C. The anionic conjugates of bilirubin and bile acids stimulate ATP hydrolysis by S-(dinitrophenyl)glutathione ATPase of human erythrocyte. FEBS Lett. 1991 Apr 9;281(1-2):255–257. doi: 10.1016/0014-5793(91)80405-r. [DOI] [PubMed] [Google Scholar]
- Tan K. H., Meyer D. J., Belin J., Ketterer B. Inhibition of microsomal lipid peroxidation by glutathione and glutathione transferases B and AA. Role of endogenous phospholipase A2. Biochem J. 1984 May 15;220(1):243–252. doi: 10.1042/bj2200243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee L. B., Gilmore K. S., Meyer D. J., Ketterer B., Vandenberghe Y., Yeoh G. C. Expression of glutathione S-transferase during rat liver development. Biochem J. 1992 Feb 15;282(Pt 1):209–218. doi: 10.1042/bj2820209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew K. D., Bomber A. M., Hoffman S. J. Ethacrynic acid and piriprost as enhancers of cytotoxicity in drug resistant and sensitive cell lines. Cancer Res. 1988 Jul 1;48(13):3622–3625. [PubMed] [Google Scholar]
- Vander Jagt D. L., Hunsaker L. A., Garcia K. B., Royer R. E. Isolation and characterization of the multiple glutathione S-transferases from human liver. Evidence for unique heme-binding sites. J Biol Chem. 1985 Sep 25;260(21):11603–11610. [PubMed] [Google Scholar]


