Abstract
The anti-pan carcinoma monoclonal antibody (MAb) 323/A3, linked to E. coli-derived beta-glucuronidase (GUS) was used to study the tumour-site-selective activation of the prodrug Epirubicin-glucuronide (Epi-glu). Epi-glu was isolated from the urine of patients treated with Epirubicin (Epi) by reversed phase chromatography on a silica-C18 column. Epi-glu was stable in human blood and was not converted into Epi by A2780, MCF-7, or OVCAR-3 cancer cells, despite the presence of intracellular GUS. The stability of the prodrug was confirmed in BALB/c mice. MAb 323/A3 and GUS were linked through a stable thioether bond. The conjugate (1:1) was purified by ion exchange and gel filtration chromatography. Binding to target cells revealed an immunoreactivity of at least 60% and good retention of enzyme activity. A protein dye (sulforhodamine B) assay was used to analyse cytotoxicity. Epi (IC50 of 0.003-0.2 microM) was 100-1,000 times more toxic than Epi-glu (IC50 of greater than 20 microM), when cancer cells were exposed for 4 or 24 h to the drugs. The low cytotoxicity of Epi-glu was most likely due to the reduced cellular uptake rate of the prodrug (2.7 pmol 10(-6) cells min-1) as compared to that of the parent compound (25 pmol 10(-6) cells min-1). Pretreatment of antigen-positive cells with the 323/A3-GUS conjugate prior to prodrug exposure completely restored cytotoxicity as a result from hydrolysis of Epi-glu into Epi. Our results demonstrate that the 323/A3-GUS conjugate can specifically activate the stable non-toxic prodrug Epi-glu at the tumour cell level.
Full text
PDF
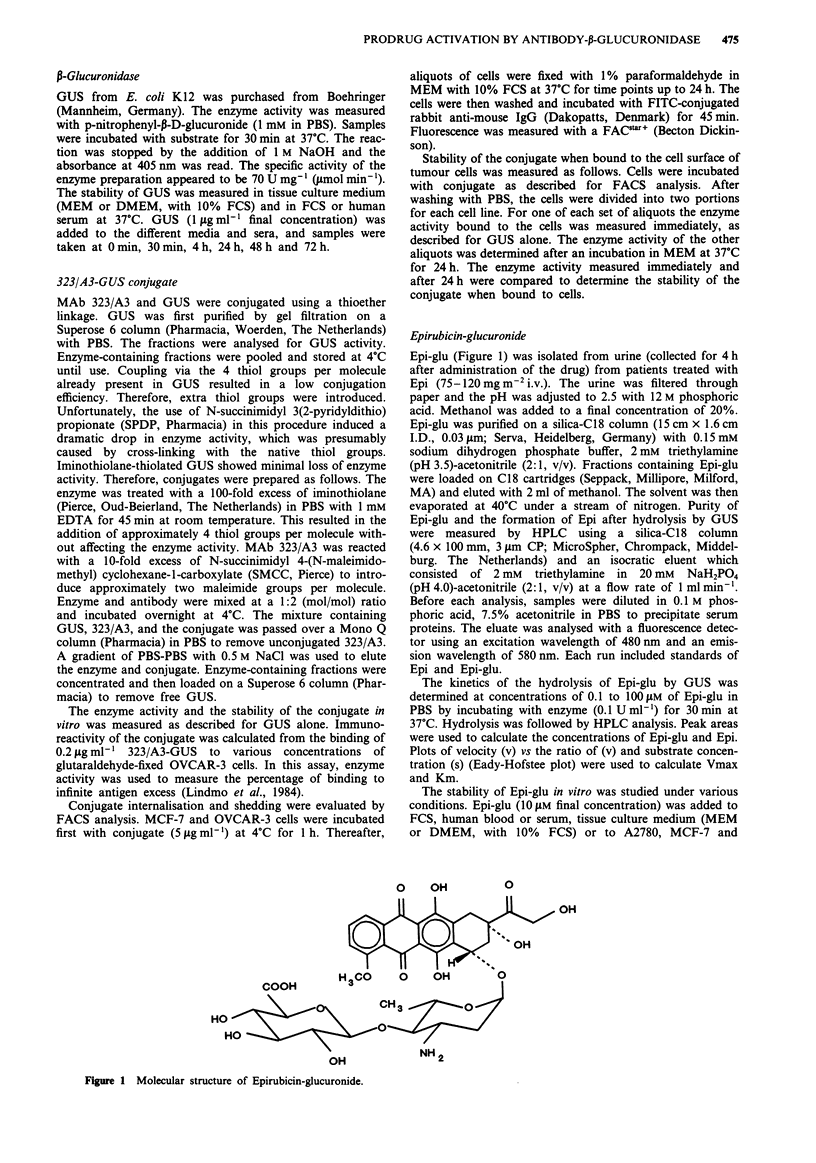
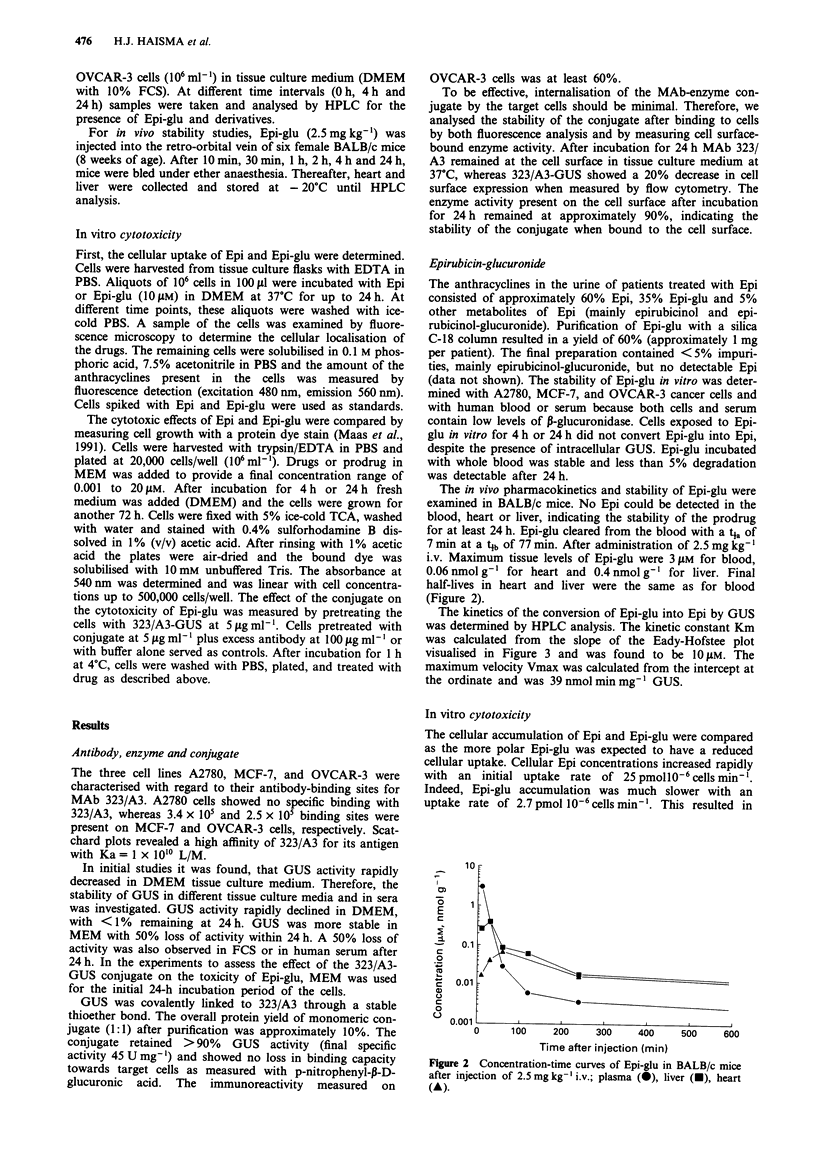
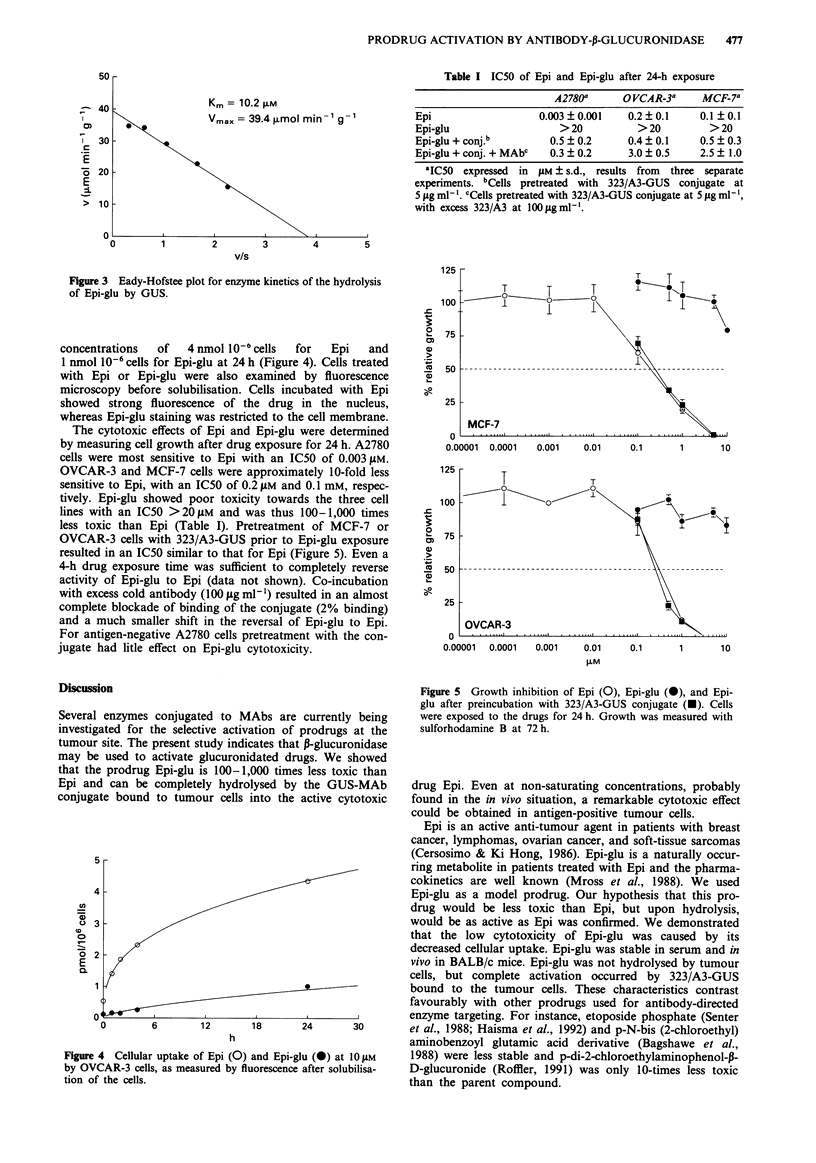

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagshawe K. D., Springer C. J., Searle F., Antoniw P., Sharma S. K., Melton R. G., Sherwood R. F. A cytotoxic agent can be generated selectively at cancer sites. Br J Cancer. 1988 Dec;58(6):700–703. doi: 10.1038/bjc.1988.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosslet K., Czech J., Lorenz P., Sedlacek H. H., Schuermann M., Seemann G. Molecular and functional characterisation of a fusion protein suited for tumour specific prodrug activation. Br J Cancer. 1992 Feb;65(2):234–238. doi: 10.1038/bjc.1992.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cersosimo R. J., Hong W. K. Epirubicin: a review of the pharmacology, clinical activity, and adverse effects of an adriamycin analogue. J Clin Oncol. 1986 Mar;4(3):425–439. doi: 10.1200/JCO.1986.4.3.425. [DOI] [PubMed] [Google Scholar]
- Edwards D. P., Grzyb K. T., Dressler L. G., Mansel R. E., Zava D. T., Sledge G. W., Jr, McGuire W. L. Monoclonal antibody identification and characterization of a Mr 43,000 membrane glycoprotein associated with human breast cancer. Cancer Res. 1986 Mar;46(3):1306–1317. [PubMed] [Google Scholar]
- Haenseler E., Esswein A., Vitols K. S., Montejano Y., Mueller B. M., Reisfeld R. A., Huennekens F. M. Activation of methotrexate-alpha-alanine by carboxypeptidase A-monoclonal antibody conjugate. Biochemistry. 1992 Jan 28;31(3):891–897. doi: 10.1021/bi00118a035. [DOI] [PubMed] [Google Scholar]
- Haisma H. J., Boven E., van Muijen M., De Vries R., Pinedo H. M. Analysis of a conjugate between anti-carcinoembryonic antigen monoclonal antibody and alkaline phosphatase for specific activation of the prodrug etoposide phosphate. Cancer Immunol Immunother. 1992;34(5):343–348. doi: 10.1007/BF01741556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haisma H. J., Hilgers J., Zurawski V. R., Jr Iodination of monoclonal antibodies for diagnosis and radiotherapy using a convenient one vial method. J Nucl Med. 1986 Dec;27(12):1890–1895. [PubMed] [Google Scholar]
- Hamilton T. C., Young R. C., Louie K. G., Behrens B. C., McKoy W. M., Grotzinger K. R., Ozols R. F. Characterization of a xenograft model of human ovarian carcinoma which produces ascites and intraabdominal carcinomatosis in mice. Cancer Res. 1984 Nov;44(11):5286–5290. [PubMed] [Google Scholar]
- Kerr D. E., Senter P. D., Burnett W. V., Hirschberg D. L., Hellström I., Hellström K. E. Antibody-penicillin-V-amidase conjugates kill antigen-positive tumor cells when combined with doxorubicin phenoxyacetamide. Cancer Immunol Immunother. 1990;31(4):202–206. doi: 10.1007/BF01789169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindmo T., Boven E., Cuttitta F., Fedorko J., Bunn P. A., Jr Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods. 1984 Aug 3;72(1):77–89. doi: 10.1016/0022-1759(84)90435-6. [DOI] [PubMed] [Google Scholar]
- Maas I. W., Boven E., Pinedo H. M., Schlüper H. M., Haisma H. J. The effects of gamma-interferon combined with 5-fluorouracil or 5-fluoro-2'-deoxyuridine on proliferation and antigen expression in a panel of human colorectal cancer cell lines. Int J Cancer. 1991 Jul 9;48(5):749–756. doi: 10.1002/ijc.2910480520. [DOI] [PubMed] [Google Scholar]
- Mross K., Maessen P., van der Vijgh W. J., Gall H., Boven E., Pinedo H. M. Pharmacokinetics and metabolism of epidoxorubicin and doxorubicin in humans. J Clin Oncol. 1988 Mar;6(3):517–526. doi: 10.1200/JCO.1988.6.3.517. [DOI] [PubMed] [Google Scholar]
- Roffler S. R., Wang S. M., Chern J. W., Yeh M. Y., Tung E. Anti-neoplastic glucuronide prodrug treatment of human tumor cells targeted with a monoclonal antibody-enzyme conjugate. Biochem Pharmacol. 1991 Oct 24;42(10):2062–2065. doi: 10.1016/0006-2952(91)90612-9. [DOI] [PubMed] [Google Scholar]
- Senter P. D., Saulnier M. G., Schreiber G. J., Hirschberg D. L., Brown J. P., Hellström I., Hellström K. E. Anti-tumor effects of antibody-alkaline phosphatase conjugates in combination with etoposide phosphate. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4842–4846. doi: 10.1073/pnas.85.13.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Tannock I. F., Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989 Aug 15;49(16):4373–4384. [PubMed] [Google Scholar]


