Abstract
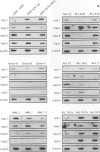
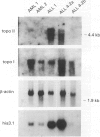
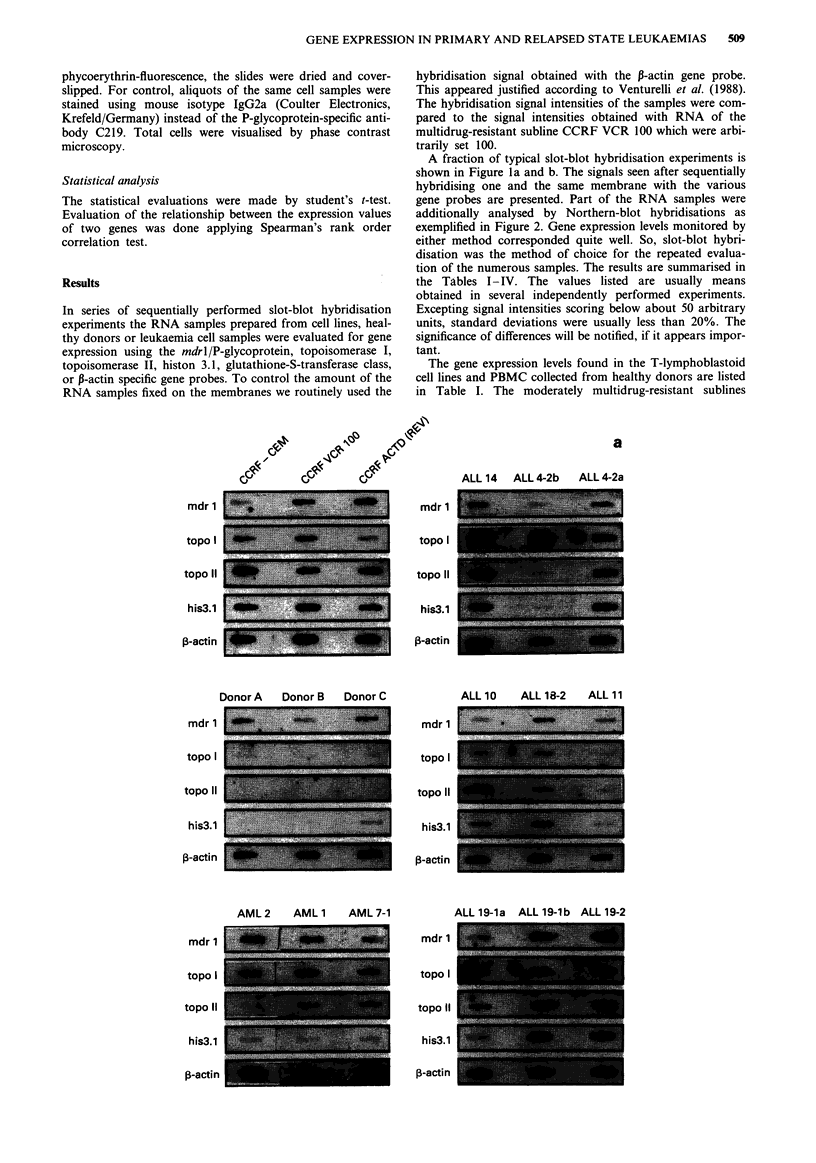
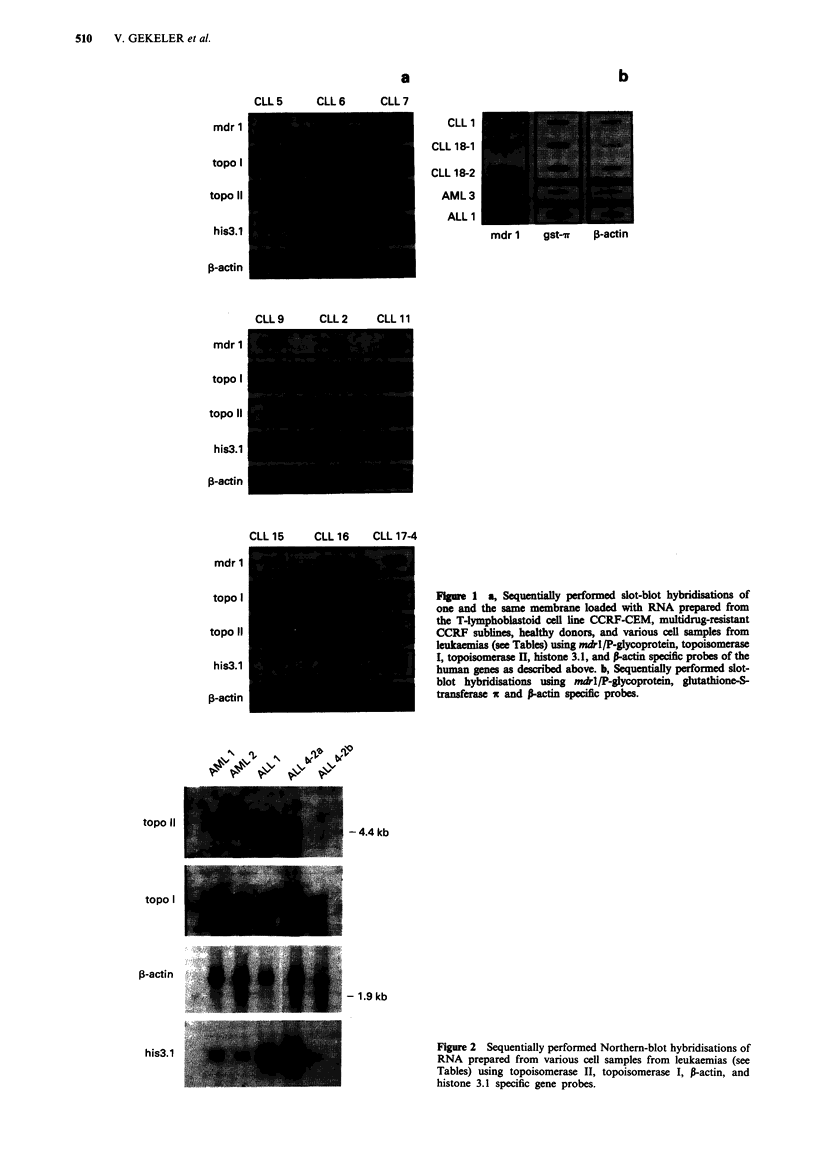
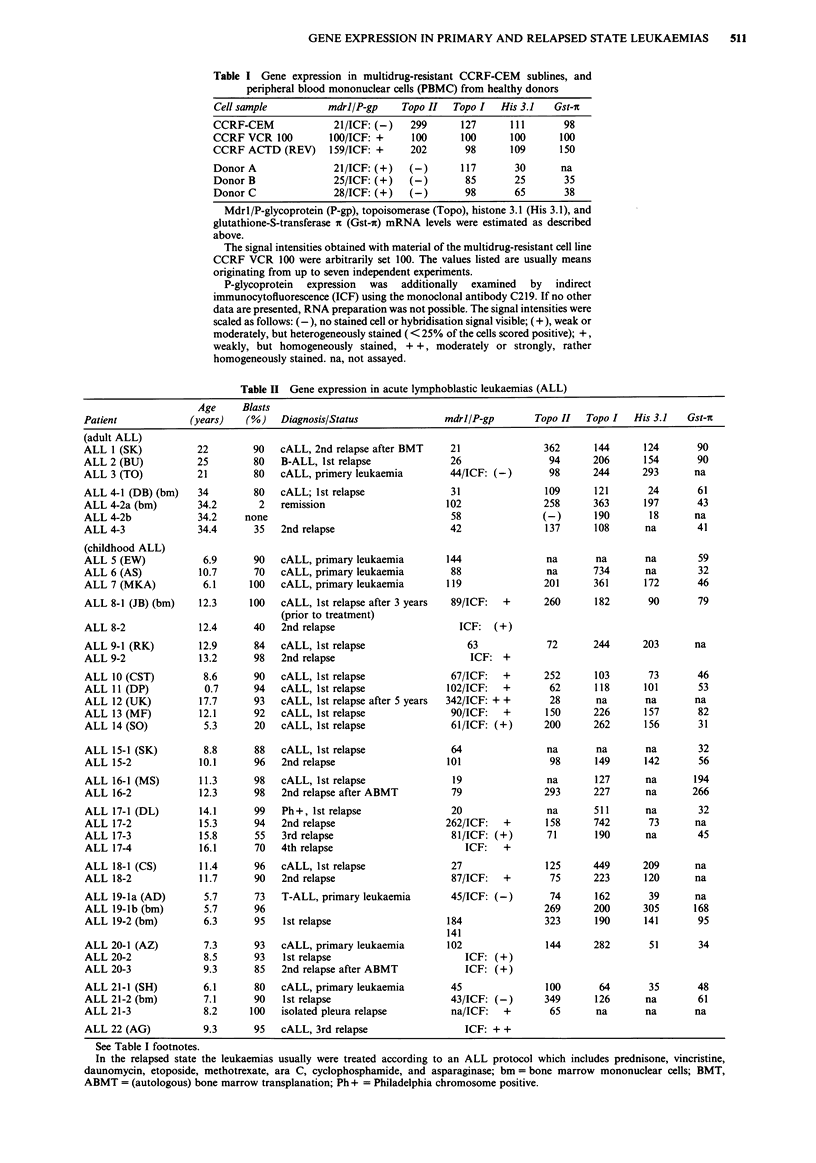
In a variety of adult and childhood leukaemia cell samples collected at different states of the disease, we analysed in a series of sequentially performed slot-blot or Northern-blot hybridisation experiments the expression of genes possibly involved in multiple drug resistance (MDR) (mdr1/P-glycoprotein, DNA topoisomerase II, glutathione-S-transferase pi), and the expression of the DNA topoisomerase I and histone 3.1 genes. Occasionally, P-glycoprotein gene expression was additionally examined by indirect immunocytofluorescence using the monoclonal antibody C219. No significant difference in mdr1/P-glycoprotein mRNA levels between primary and relapsed state acute lymphocytic leukaemias (ALL) was seen on average. Second or third relapses, however, showed a distinct tendency to an elevated expression of this multidrug transporter gene (up to 10-fold) in part well beyond the value seen in the moderately cross-resistant T-lymphoblastoid CCRF-CEM subline CCRF VCR 100. Increased mdr1/P-glycoprotein mRNA levels were also found in relapsed state acute myelogenous leukaemias (AML), and in chronic lymphocytic leukaemias (CLL) treated with chlorambucil and/or prednisone for several years. Topoisomerase I and topoisomerase II mRNA levels were found to be very variable. Whereas in all but one case of CLL topoisomerase II mRNA was not detected by slot-blot hybridizations, strong topoisomerase I and topoisomerase II gene expression levels, frequently exceeding the levels monitored in the CCRF-CEM cell line, were seen in many cell samples of acute leukaemia. If topoisomerase II mRNA was undetectable, expression of topoisomerase I was clearly visible throughout. These observations might be valuable considering the possible treatment with specific topoisomerase I or topoisomerase II inhibitors. Significant positive correlations were found (i) for topoisomerase I and histone 3.1 gene expression levels in general (P less than 0.001), and (ii) in the CLL samples additionally for the expression levels of the mdr1 gene, and the histone 3.1, topoisomerase I, and glutathione-S-transferase pi genes, respectively.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arceci R. J., Croop J. M., Horwitz S. B., Housman D. The gene encoding multidrug resistance is induced and expressed at high levels during pregnancy in the secretory epithelium of the uterus. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4350–4354. doi: 10.1073/pnas.85.12.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Burt R. K., Thorgeirsson S. S. Coinduction of MDR-1 multidrug-resistance and cytochrome P-450 genes in rat liver by xenobiotics. J Natl Cancer Inst. 1988 Nov 2;80(17):1383–1386. doi: 10.1093/jnci/80.17.1383. [DOI] [PubMed] [Google Scholar]
- Chen A. Y., Yu C., Potmesil M., Wall M. E., Wani M. C., Liu L. F. Camptothecin overcomes MDR1-mediated resistance in human KB carcinoma cells. Cancer Res. 1991 Nov 15;51(22):6039–6044. [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dalton W. S., Grogan T. M., Meltzer P. S., Scheper R. J., Durie B. G., Taylor C. W., Miller T. P., Salmon S. E. Drug-resistance in multiple myeloma and non-Hodgkin's lymphoma: detection of P-glycoprotein and potential circumvention by addition of verapamil to chemotherapy. J Clin Oncol. 1989 Apr;7(4):415–424. doi: 10.1200/JCO.1989.7.4.415. [DOI] [PubMed] [Google Scholar]
- Davies S. M., Robson C. N., Davies S. L., Hickson I. D. Nuclear topoisomerase II levels correlate with the sensitivity of mammalian cells to intercalating agents and epipodophyllotoxins. J Biol Chem. 1988 Nov 25;263(33):17724–17729. [PubMed] [Google Scholar]
- Deffie A. M., Alam T., Seneviratne C., Beenken S. W., Batra J. K., Shea T. C., Henner W. D., Goldenberg G. J. Multifactorial resistance to adriamycin: relationship of DNA repair, glutathione transferase activity, drug efflux, and P-glycoprotein in cloned cell lines of adriamycin-sensitive and -resistant P388 leukemia. Cancer Res. 1988 Jul 1;48(13):3595–3602. [PubMed] [Google Scholar]
- Drake F. H., Hofmann G. A., Bartus H. F., Mattern M. R., Crooke S. T., Mirabelli C. K. Biochemical and pharmacological properties of p170 and p180 forms of topoisomerase II. Biochemistry. 1989 Oct 3;28(20):8154–8160. doi: 10.1021/bi00446a029. [DOI] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Fairchild C. R., Ivy S. P., Rushmore T., Lee G., Koo P., Goldsmith M. E., Myers C. E., Farber E., Cowan K. H. Carcinogen-induced mdr overexpression is associated with xenobiotic resistance in rat preneoplastic liver nodules and hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7701–7705. doi: 10.1073/pnas.84.21.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild C. R., Moscow J. A., O'Brien E. E., Cowan K. H. Multidrug resistance in cells transfected with human genes encoding a variant P-glycoprotein and glutathione S-transferase-pi. Mol Pharmacol. 1990 Jun;37(6):801–809. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fernandes D. J., Danks M. K., Beck W. T. Decreased nuclear matrix DNA topoisomerase II in human leukemia cells resistant to VM-26 and m-AMSA. Biochemistry. 1990 May 1;29(17):4235–4241. doi: 10.1021/bi00469a028. [DOI] [PubMed] [Google Scholar]
- Finstad C. L., Yin B. W., Gordon C. M., Federici M. G., Welt S., Lloyd K. O. Some monoclonal antibody reagents (C219 and JSB-1) to P-glycoprotein contain antibodies to blood group A carbohydrate determinants: a problem of quality control for immunohistochemical analysis. J Histochem Cytochem. 1991 Dec;39(12):1603–1610. doi: 10.1177/39.12.1682363. [DOI] [PubMed] [Google Scholar]
- Fojo A. T., Shen D. W., Mickley L. A., Pastan I., Gottesman M. M. Intrinsic drug resistance in human kidney cancer is associated with expression of a human multidrug-resistance gene. J Clin Oncol. 1987 Dec;5(12):1922–1927. doi: 10.1200/JCO.1987.5.12.1922. [DOI] [PubMed] [Google Scholar]
- Gekeler V., Frese G., Diddens H., Probst H. Expression of a P-glycoprotein gene is inducible in a multidrug-resistant human leukemia cell line. Biochem Biophys Res Commun. 1988 Sep 15;155(2):754–760. doi: 10.1016/s0006-291x(88)80559-x. [DOI] [PubMed] [Google Scholar]
- Gekeler V., Weger S., Probst H. mdr1/P-glycoprotein gene segments analyzed from various human leukemic cell lines exhibiting different multidrug resistance profiles. Biochem Biophys Res Commun. 1990 Jun 15;169(2):796–802. doi: 10.1016/0006-291x(90)90401-8. [DOI] [PubMed] [Google Scholar]
- Giovanella B. C., Stehlin J. S., Wall M. E., Wani M. C., Nicholas A. W., Liu L. F., Silber R., Potmesil M. DNA topoisomerase I--targeted chemotherapy of human colon cancer in xenografts. Science. 1989 Nov 24;246(4933):1046–1048. doi: 10.1126/science.2555920. [DOI] [PubMed] [Google Scholar]
- Goldie J. H., Coldman A. J. The genetic origin of drug resistance in neoplasms: implications for systemic therapy. Cancer Res. 1984 Sep;44(9):3643–3653. [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker W. G., Slade D. L., Dalton W. S., Meltzer P. S., Trent J. M. Multidrug resistance in mitoxantrone-selected HL-60 leukemia cells in the absence of P-glycoprotein overexpression. Cancer Res. 1989 Aug 15;49(16):4542–4549. [PubMed] [Google Scholar]
- Herweijer H., Sonneveld P., Baas F., Nooter K. Expression of mdr1 and mdr3 multidrug-resistance genes in human acute and chronic leukemias and association with stimulation of drug accumulation by cyclosporine. J Natl Cancer Inst. 1990 Jul 4;82(13):1133–1140. doi: 10.1093/jnci/82.13.1133. [DOI] [PubMed] [Google Scholar]
- Holden J. A., Rolfson D. H., Wittwer C. T. Human DNA topoisomerase II: evaluation of enzyme activity in normal and neoplastic tissues. Biochemistry. 1990 Feb 27;29(8):2127–2134. doi: 10.1021/bi00460a024. [DOI] [PubMed] [Google Scholar]
- Holmes J. A., Jacobs A., Carter G., Whittaker J. A., Bentley D. P., Padua R. A. Is the mdr 1 gene relevant in chronic lymphocytic leukemia? Leukemia. 1990 Mar;4(3):216–218. [PubMed] [Google Scholar]
- Holmes J., Wareing C., Jacobs A., Hayes J. D., Padua R. A., Wolf C. R. Glutathione-s-transferase pi expression in leukaemia: a comparative analysis with mdr-1 data. Br J Cancer. 1990 Aug;62(2):209–212. doi: 10.1038/bjc.1990.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwong C. L., Wang C. H., Chen Y. J., Whang-Peng J., Hwang J. L. Induction of topoisomerase II gene expression in human lymphocytes upon phytohemagglutinin stimulation. Cancer Res. 1990 Sep 1;50(17 Suppl):5649S–5652S. [PubMed] [Google Scholar]
- Ito Y., Tanimoto M., Kumazawa T., Okumura M., Morishima Y., Ohno R., Saito H. Increased P-glycoprotein expression and multidrug-resistant gene (mdr1) amplification are infrequently found in fresh acute leukemia cells. Sequential analysis of 15 cases at initial presentation and relapsed stage. Cancer. 1989 Apr 15;63(8):1534–1538. doi: 10.1002/1097-0142(19890415)63:8<1534::aid-cncr2820630813>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Kano T., Sakai M., Muramatsu M. Structure and expression of a human class pi glutathione S-transferase messenger RNA. Cancer Res. 1987 Nov 1;47(21):5626–5630. [PubMed] [Google Scholar]
- Kim R., Hirabayashi N., Nishiyama M., Saeki S., Toge T., Okada K. Expression of MDR1, GST-pi and topoisomerase II as an indicator of clinical response to adriamycin. Anticancer Res. 1991 Jan-Feb;11(1):429–431. [PubMed] [Google Scholar]
- Kimmig A., Gekeler V., Neumann M., Frese G., Handgretinger R., Kardos G., Diddens H., Niethammer D. Susceptibility of multidrug-resistant human leukemia cell lines to human interleukin 2-activated killer cells. Cancer Res. 1990 Nov 1;50(21):6793–6799. [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Ma D. D., Scurr R. D., Davey R. A., Mackertich S. M., Harman D. H., Dowden G., Isbister J. P., Bell D. R. Detection of a multidrug resistant phenotype in acute non-lymphoblastic leukaemia. Lancet. 1987 Jan 17;1(8525):135–137. doi: 10.1016/s0140-6736(87)91969-6. [DOI] [PubMed] [Google Scholar]
- Madden K. R., Champoux J. J. Overexpression of human topoisomerase I in baby hamster kidney cells: hypersensitivity of clonal isolates to camptothecin. Cancer Res. 1992 Feb 1;52(3):525–532. [PubMed] [Google Scholar]
- McGrath T., Center M. S. Mechanisms of multidrug resistance in HL60 cells: evidence that a surface membrane protein distinct from P-glycoprotein contributes to reduced cellular accumulation of drug. Cancer Res. 1988 Jul 15;48(14):3959–3963. [PubMed] [Google Scholar]
- McQuaid S., McCann S., Daly P., Lawlor E., Humphries P. Observations on the transcriptional activity of the glutathione S-transferase pi gene in human haematological malignancies and in the peripheral leucocytes of cancer patients under chemotherapy. Br J Cancer. 1989 Apr;59(4):540–543. doi: 10.1038/bjc.1989.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley L. A., Bates S. E., Richert N. D., Currier S., Tanaka S., Foss F., Rosen N., Fojo A. T. Modulation of the expression of a multidrug resistance gene (mdr-1/P-glycoprotein) by differentiating agents. J Biol Chem. 1989 Oct 25;264(30):18031–18040. [PubMed] [Google Scholar]
- Musto P., Cascavilla N., Di Renzo N., Ladogana S., La Sala A., Melillo L., Nobile M., Matera R., Lombardi G., Carotenuto M. Clinical relevance of immunocytochemical detection of multidrug-resistance-associated P-glycoprotein in hematologic malignancies. Tumori. 1990 Aug 31;76(4):353–359. doi: 10.1177/030089169007600410. [DOI] [PubMed] [Google Scholar]
- Niethammer D., Diddens H., Gekeler V., Frese G., Handgretinger R., Henze G., Schmidt H., Probst H. Resistance to methotrexate and multidrug resistance in childhood malignancies. Adv Enzyme Regul. 1989;29:231–245. doi: 10.1016/0065-2571(89)90104-0. [DOI] [PubMed] [Google Scholar]
- Noonan K. E., Beck C., Holzmayer T. A., Chin J. E., Wunder J. S., Andrulis I. L., Gazdar A. F., Willman C. L., Griffith B., Von Hoff D. D. Quantitative analysis of MDR1 (multidrug resistance) gene expression in human tumors by polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7160–7164. doi: 10.1073/pnas.87.18.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker R., Wallner J., Geissler K., Linkesch W., Haas O. A., Bettelheim P., Hopfner M., Scherrer R., Valent P., Havelec L. MDR1 gene expression and treatment outcome in acute myeloid leukemia. J Natl Cancer Inst. 1991 May 15;83(10):708–712. doi: 10.1093/jnci/83.10.708. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Kerrigan D., Schwartz R. E., Swack J. A., McCurdy A. Altered DNA topoisomerase II activity in Chinese hamster cells resistant to topoisomerase II inhibitors. Cancer Res. 1986 Jun;46(6):3075–3081. [PubMed] [Google Scholar]
- Romig H., Richter A. Expression of the topoisomerase I gene in serum stimulated human fibroblasts. Biochim Biophys Acta. 1990 Apr 6;1048(2-3):274–280. doi: 10.1016/0167-4781(90)90067-c. [DOI] [PubMed] [Google Scholar]
- Schinkel A. H., Roelofs E. M., Borst P. Characterization of the human MDR3 P-glycoprotein and its recognition by P-glycoprotein-specific monoclonal antibodies. Cancer Res. 1991 May 15;51(10):2628–2635. [PubMed] [Google Scholar]
- Schlaifer D., Laurent G., Chittal S., Tsuruo T., Soues S., Muller C., Charcosset J. Y., Alard C., Brousset P., Mazerrolles C. Immunohistochemical detection of multidrug resistance associated P-glycoprotein in tumour and stromal cells of human cancers. Br J Cancer. 1990 Aug;62(2):177–182. doi: 10.1038/bjc.1990.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber R., Potmesil M., Bank B. B. Studies on drug resistance in chronic lymphocytic leukemia. Adv Enzyme Regul. 1989;29:267–276. doi: 10.1016/0065-2571(89)90106-4. [DOI] [PubMed] [Google Scholar]
- Sullivan D. M., Latham M. D., Ross W. E. Proliferation-dependent topoisomerase II content as a determinant of antineoplastic drug action in human, mouse, and Chinese hamster ovary cells. Cancer Res. 1987 Aug 1;47(15):3973–3979. [PubMed] [Google Scholar]
- Tsai-Pflugfelder M., Liu L. F., Liu A. A., Tewey K. M., Whang-Peng J., Knutsen T., Huebner K., Croce C. M., Wang J. C. Cloning and sequencing of cDNA encoding human DNA topoisomerase II and localization of the gene to chromosome region 17q21-22. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7177–7181. doi: 10.1073/pnas.85.19.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubezio P., Limonta M., D'Incalci M., Damia G., Masera G., Giudici G., Wolverton J. S., Beck W. T. Failure to detect the P-glycoprotein multidrug resistant phenotype in cases of resistant childhood acute lymphocytic leukaemia. Eur J Cancer Clin Oncol. 1989 Dec;25(12):1895–1899. doi: 10.1016/0277-5379(89)90367-2. [DOI] [PubMed] [Google Scholar]
- Venturelli D., Lange B., Narni F., Selleri L., Mariano M. T., Torelli U., Gewirtz A. M., Calabretta B. Prognostic significance of "short-term" effects of chemotherapy on MYC and histone H3 mRNA levels in acute leukemia patients. Proc Natl Acad Sci U S A. 1988 May;85(10):3590–3594. doi: 10.1073/pnas.85.10.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volm M., Efferth T., Bak M., Ho A. D., Mattern J. Detection of the multidrug resistant phenotype in human tumours by monoclonal antibodies and the streptavidin-biotinylated phycoerythrin complex method. Eur J Cancer Clin Oncol. 1989 Apr;25(4):743–749. doi: 10.1016/0277-5379(89)90213-7. [DOI] [PubMed] [Google Scholar]
- Waxman D. J. Glutathione S-transferases: role in alkylating agent resistance and possible target for modulation chemotherapy--a review. Cancer Res. 1990 Oct 15;50(20):6449–6454. [PubMed] [Google Scholar]
- de Jong S., Zijlstra J. G., de Vries E. G., Mulder N. H. Reduced DNA topoisomerase II activity and drug-induced DNA cleavage activity in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res. 1990 Jan 15;50(2):304–309. [PubMed] [Google Scholar]