Abstract
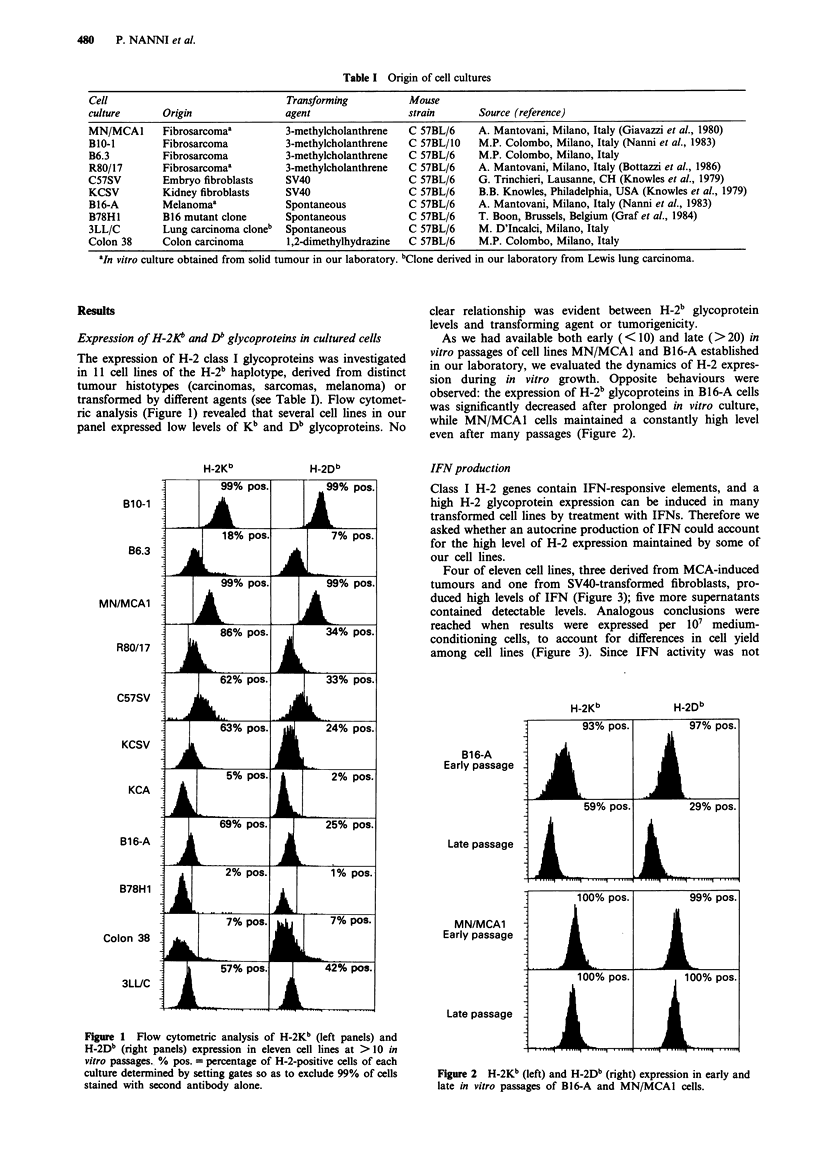
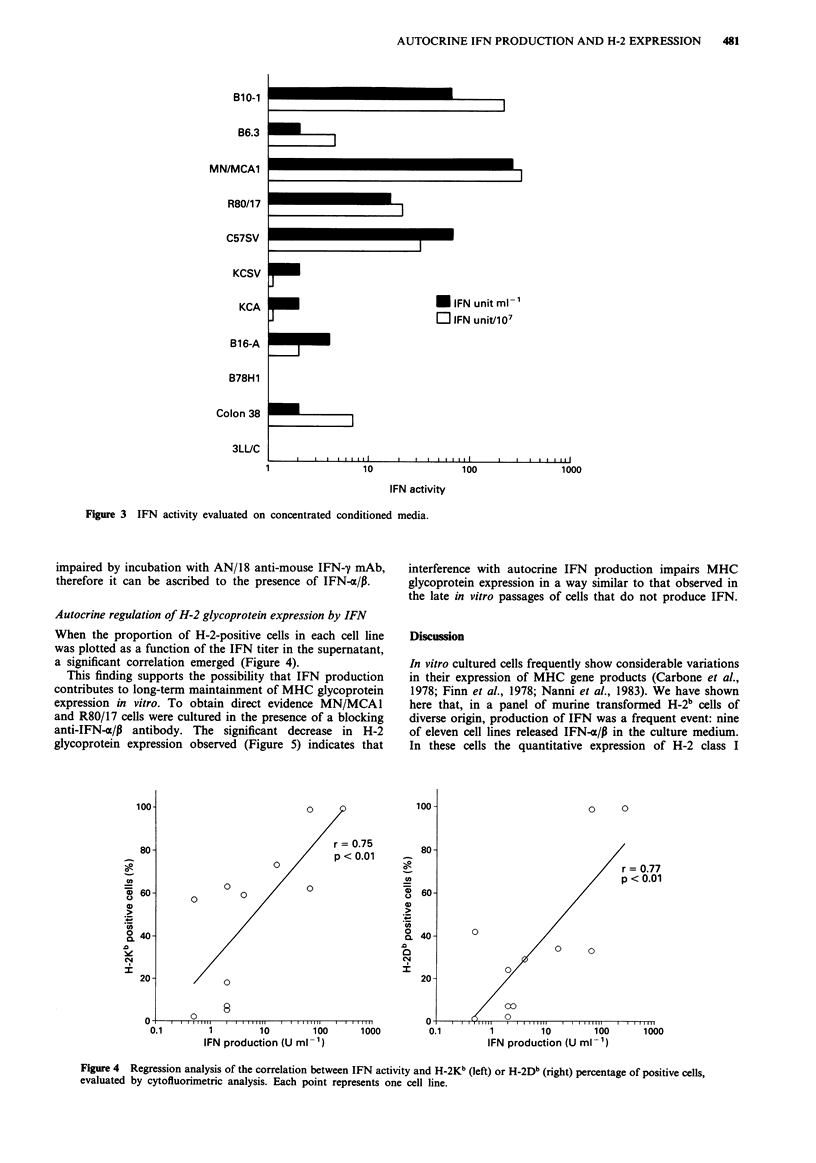
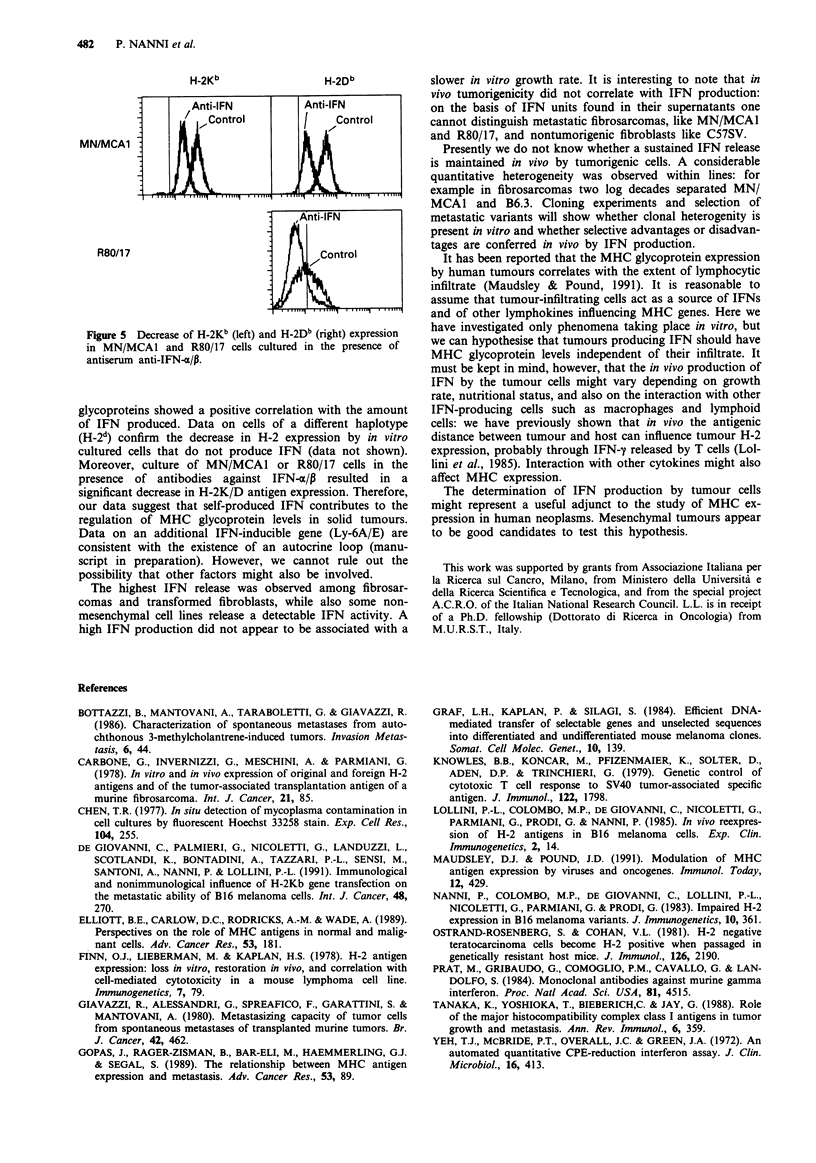
The relationship between autocrine interferon (IFN) production and the expression of class I Major Histocompatibility Complex (MHC) membrane glycoproteins in vitro was investigated in a panel of murine transformed cells of nonhaemopoietic origin. The panel included 11 cell lines of H-2Kb haplotype derived from fibrosarcomas, carcinomas and melanoma, and from transformed fibroblasts. IFN activity was detected in the conditioned medium of nine cell lines; fibrosarcomas were among the high IFN producers, while the non-producers were a melanoma clone and a lung carcinoma cell line. A significant correlation was found between IFN production and the expression of H-2K/D glycoproteins, thus suggesting that long-term maintainment of MHC glycoprotein expression in vitro could be mediated by self produced IFN. Two IFN producer cell lines, MN/MCA1 and R80/17, were cultured in the presence of a blocking antiserum against IFN-alpha/beta: a significant decrease in H-2b expression was observed, thus indicating the existence of an autocrine IFN circuit. Taken together these findings suggest that release of IFN is a frequent event among transformed nonhaemopoietic cells, and that self-produced IFN contributes to the regulation of MHC antigen levels in solid tumours.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottazzi B., Mantovani A., Taraboletti G., Giavazzi R. Characterization of spontaneous metastases from autochthonous 3-methylcholanthrene-induced tumors. Invasion Metastasis. 1986;6(1):44–57. [PubMed] [Google Scholar]
- Carbone G., Invernizzi G., Meschini A., Parmiani G. In vitro and in vivo expression of original and foreign H-2 antigens and of the tumor-associated transplantation antigen of a murine fibrosarcoma. Int J Cancer. 1978 Jan 15;21(1):85–93. doi: 10.1002/ijc.2910210115. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- De Giovanni C., Palmieri G., Nicoletti G., Landuzzi L., Scotlandi K., Bontadini A., Tazzari P. L., Sensi M., Santoni A., Nanni P. Immunological and non-immunological influence of H-2Kb gene transfection on the metastatic ability of B16 melanoma cells. Int J Cancer. 1991 May 10;48(2):270–276. doi: 10.1002/ijc.2910480220. [DOI] [PubMed] [Google Scholar]
- Elliott B. E., Carlow D. A., Rodricks A. M., Wade A. Perspectives on the role of MHC antigens in normal and malignant cell development. Adv Cancer Res. 1989;53:181–245. doi: 10.1016/s0065-230x(08)60282-1. [DOI] [PubMed] [Google Scholar]
- Giavazzi R., Alessandri G., Spreafico F., Garattini S., Mantovani A. Metastasizing capacity of tumour cells from spontaneous metastases of transplanted murine tumours. Br J Cancer. 1980 Sep;42(3):462–472. doi: 10.1038/bjc.1980.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopas J., Rager-Zisman B., Bar-Eli M., Hämmerling G. J., Segal S. The relationship between MHC antigen expression and metastasis. Adv Cancer Res. 1989;53:89–115. doi: 10.1016/s0065-230x(08)60280-8. [DOI] [PubMed] [Google Scholar]
- Graf L. H., Jr, Kaplan P., Silagi S. Efficient DNA-mediated transfer of selectable genes and unselected sequences into differentiated and undifferentiated mouse melanoma clones. Somat Cell Mol Genet. 1984 Mar;10(2):139–151. doi: 10.1007/BF01534903. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Koncar M., Pfizenmaier K., Solter D., Aden D. P., Trinchieri G. Genetic control of the cytotoxic T cell response to SV40 tumor-associated specific antigen. J Immunol. 1979 May;122(5):1798–1806. [PubMed] [Google Scholar]
- Lollini P. L., Colombo M. P., De Giovanni C., Nicoletti G., Parmiani G., Prodi G., Nanni P. In vivo reexpression of H-2 antigens in B16 melanoma cells. Exp Clin Immunogenet. 1985;2(1):14–23. [PubMed] [Google Scholar]
- Maudsley D. J., Pound J. D. Modulation of MHC antigen expression by viruses and oncogenes. Immunol Today. 1991 Dec;12(12):429–431. doi: 10.1016/0167-5699(91)90013-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni P., Colombo M. P., De Giovanni C., Lollini P. L., Nicoletti G., Parmiani G., Prodi G. Impaired H-2 expression in B16 melanoma variants. J Immunogenet. 1983 Oct;10(5):361–370. doi: 10.1111/j.1744-313x.1983.tb00348.x. [DOI] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S., Cohan V. L. H-2 negative teratocarcinoma cells become H-2 positive when passaged in genetically resistant host mice. J Immunol. 1981 Jun;126(6):2190–2193. [PubMed] [Google Scholar]
- Prat M., Gribaudo G., Comoglio P. M., Cavallo G., Landolfo S. Monoclonal antibodies against murine gamma interferon. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4515–4519. doi: 10.1073/pnas.81.14.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Yoshioka T., Bieberich C., Jay G. Role of the major histocompatibility complex class I antigens in tumor growth and metastasis. Annu Rev Immunol. 1988;6:359–380. doi: 10.1146/annurev.iy.06.040188.002043. [DOI] [PubMed] [Google Scholar]
- Yeh T. J., McBride P. T., Overall J. C., Jr, Green J. A. Automated, quantitative cytopathic effect reduction assay for interferon. J Clin Microbiol. 1982 Aug;16(2):413–415. doi: 10.1128/jcm.16.2.413-415.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


