Abstract
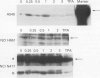
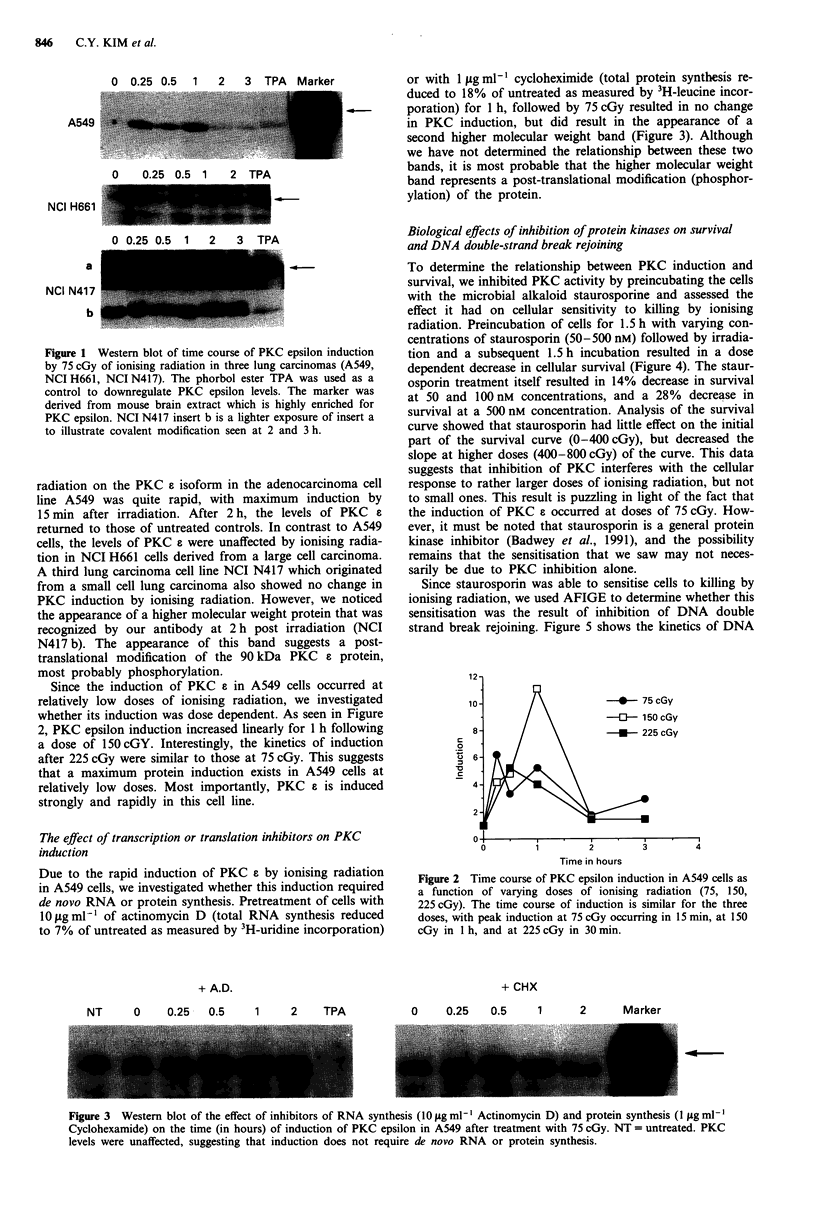
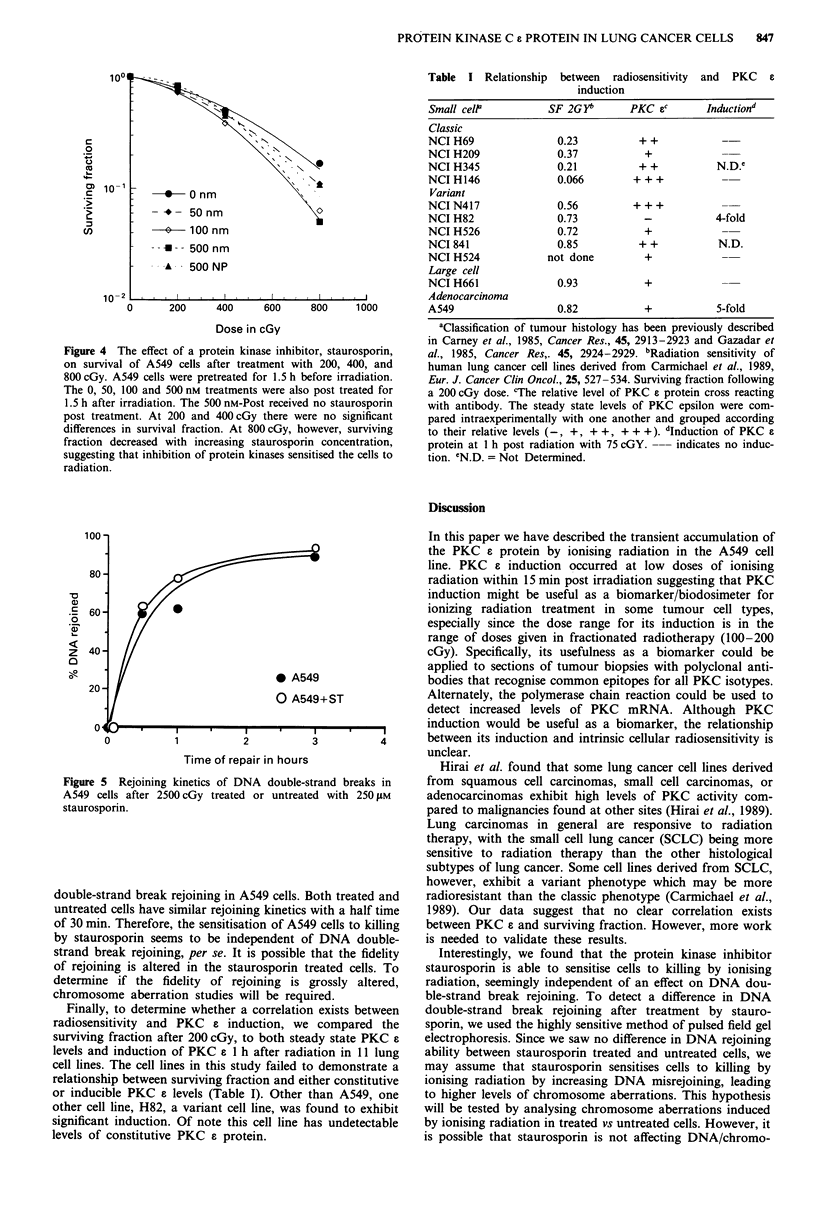
The effect of ionising radiation on the regulation of gene and protein expression is complex. This study focuses on the translational regulational of the epsilon isoform of protein kinase C by ionising radiation. We found that protein kinase C epsilon is rapidly increased in the human lung adenocarcinoma cell line A549 following irradiation. Western blots showed increased accumulation of this protein at doses as low as 75 cGy after 15 min post irradiation. Maximal induction (11-fold over unirradiated cells) of PKC epsilon occurred at 150 cGy within 1 h after treatment by X-rays in A549 cells. The increased levels of PKC epsilon protein after X-rays does not require de novo protein or RNA synthesis, suggesting that this increase is post-translationally controlled. In contrast to A549 cells PKC epsilon levels in the large cell lung carcinoma cell line NCI H661 were not induced by radiation. In the small cell lung carcinoma cell line NCI N417, PKC epsilon was also not induced but a higher molecular weight PKC epsilon protein, suggestive of phosphorylation, appeared at 2 h after irradiation. The variation in induction or phosphorylation of PKC epsilon by ionising radiation in the cell lines tested in this study suggested that no clear correlation existed between intrinsic radiation sensitivity and PKC epsilon induction. To determine whether PKC epsilon does play a role in cell survival to irradiation, we used the protein kinase inhibitor staurosporin to decrease PKC activity and found that staurosporin sensitised cells to killing by ionising radiation. Pulsed field gel electrophoresis, however, indicated that DNA double-strand break repair was not decreased, suggesting that PKC epsilon is modifying the fidelity of rejoining and not the overall magnitude of repair. The regulation of PKC by ionising radiation will be discussed with respect to the biological consequences of gene induction by DNA damage agents.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Erickson R. W., Curnutte J. T. Staurosporine inhibits the soluble and membrane-bound protein tyrosine kinases of human neutrophils. Biochem Biophys Res Commun. 1991 Jul 31;178(2):423–429. doi: 10.1016/0006-291x(91)90124-p. [DOI] [PubMed] [Google Scholar]
- Boothman D. A., Bouvard I., Hughes E. N. Identification and characterization of X-ray-induced proteins in human cells. Cancer Res. 1989 Jun 1;49(11):2871–2878. [PubMed] [Google Scholar]
- Carmichael J., Degraff W. G., Gamson J., Russo D., Gazdar A. F., Levitt M. L., Minna J. D., Mitchell J. B. Radiation sensitivity of human lung cancer cell lines. Eur J Cancer Clin Oncol. 1989 Mar;25(3):527–534. doi: 10.1016/0277-5379(89)90266-6. [DOI] [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Bepler G., Guccion J. G., Marangos P. J., Moody T. W., Zweig M. H., Minna J. D. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 1985 Jun;45(6):2913–2923. [PubMed] [Google Scholar]
- Coussens L., Parker P. J., Rhee L., Yang-Feng T. L., Chen E., Waterfield M. D., Francke U., Ullrich A. Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science. 1986 Aug 22;233(4766):859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- Denko N., Giaccia A., Peters B., Stamato T. D. An asymmetric field inversion gel electrophoresis method for the separation of large DNA molecules. Anal Biochem. 1989 Apr;178(1):172–176. doi: 10.1016/0003-2697(89)90375-8. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Alamo I., Jr, Hollander M. C. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8800–8804. doi: 10.1073/pnas.85.23.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry M. J., Gebhardt A., Parker P. J., Foulkes J. G. Phosphatidylinositol turnover and transformation of cells by Abelson murine leukaemia virus. EMBO J. 1985 Dec 1;4(12):3173–3178. doi: 10.1002/j.1460-2075.1985.tb04061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Nau M. M., Minna J. D. Characterization of variant subclasses of cell lines derived from small cell lung cancer having distinctive biochemical, morphological, and growth properties. Cancer Res. 1985 Jun;45(6):2924–2930. [PubMed] [Google Scholar]
- Giaccia A. J., Denko N., MacLaren R., Mirman D., Waldren C., Hart I., Stamato T. D. Human chromosome 5 complements the DNA double-strand break-repair deficiency and gamma-ray sensitivity of the XR-1 hamster variant. Am J Hum Genet. 1990 Sep;47(3):459–469. [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Hallahan D. E., Sukhatme V. P., Sherman M. L., Virudachalam S., Kufe D., Weichselbaum R. R. Protein kinase C mediates x-ray inducibility of nuclear signal transducers EGR1 and JUN. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2156–2160. doi: 10.1073/pnas.88.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan D. E., Virudachalam S., Sherman M. L., Huberman E., Kufe D. W., Weichselbaum R. R. Tumor necrosis factor gene expression is mediated by protein kinase C following activation by ionizing radiation. Cancer Res. 1991 Sep 1;51(17):4565–4569. [PubMed] [Google Scholar]
- Heidenreich K. A., Toledo S. P., Brunton L. L., Watson M. J., Daniel-Issakani S., Strulovici B. Insulin stimulates the activity of a novel protein kinase C, PKC-epsilon, in cultured fetal chick neurons. J Biol Chem. 1990 Sep 5;265(25):15076–15082. [PubMed] [Google Scholar]
- Hirai M., Gamou S., Kobayashi M., Shimizu N. Lung cancer cells often express high levels of protein kinase C activity. Jpn J Cancer Res. 1989 Mar;80(3):204–208. doi: 10.1111/j.1349-7006.1989.tb02292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander M. C., Fornace A. J., Jr Induction of fos RNA by DNA-damaging agents. Cancer Res. 1989 Apr 1;49(7):1687–1692. [PubMed] [Google Scholar]
- Huang K. P., Chan K. F., Singh T. J., Nakabayashi H., Huang F. L. Autophosphorylation of rat brain Ca2+-activated and phospholipid-dependent protein kinase. J Biol Chem. 1986 Sep 15;261(26):12134–12140. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Molina C. A., Ashendel C. L. Tumor promoter 12-O-tetradecanoylphorbol-13-acetate and sn-1,2-dioctanoylglycerol increase the phosphorylation of protein kinase C in cells. Cancer Res. 1991 Sep 1;51(17):4624–4630. [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Pfeffer L. M., Eisenkraft B. L., Reich N. C., Improta T., Baxter G., Daniel-Issakani S., Strulovici B. Transmembrane signaling by interferon alpha involves diacylglycerol production and activation of the epsilon isoform of protein kinase C in Daudi cells. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7988–7992. doi: 10.1073/pnas.88.18.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M. L., Datta R., Hallahan D. E., Weichselbaum R. R., Kufe D. W. Ionizing radiation regulates expression of the c-jun protooncogene. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5663–5666. doi: 10.1073/pnas.87.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strulovici B., Daniel-Issakani S., Baxter G., Knopf J., Sultzman L., Cherwinski H., Nestor J., Jr, Webb D. R., Ransom J. Distinct mechanisms of regulation of protein kinase C epsilon by hormones and phorbol diesters. J Biol Chem. 1991 Jan 5;266(1):168–173. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Inducible DNA repair systems. Annu Rev Biochem. 1985;54:425–457. doi: 10.1146/annurev.bi.54.070185.002233. [DOI] [PubMed] [Google Scholar]
- Wolff S., Afzal V., Wiencke J. K., Olivieri G., Michaeli A. Human lymphocytes exposed to low doses of ionizing radiations become refractory to high doses of radiation as well as to chemical mutagens that induce double-strand breaks in DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 1988 Jan;53(1):39–47. doi: 10.1080/09553008814550401. [DOI] [PubMed] [Google Scholar]
- Woloschak G. E., Chang-Liu C. M., Shearin-Jones P. Regulation of protein kinase C by ionizing radiation. Cancer Res. 1990 Jul 1;50(13):3963–3967. [PubMed] [Google Scholar]