Abstract
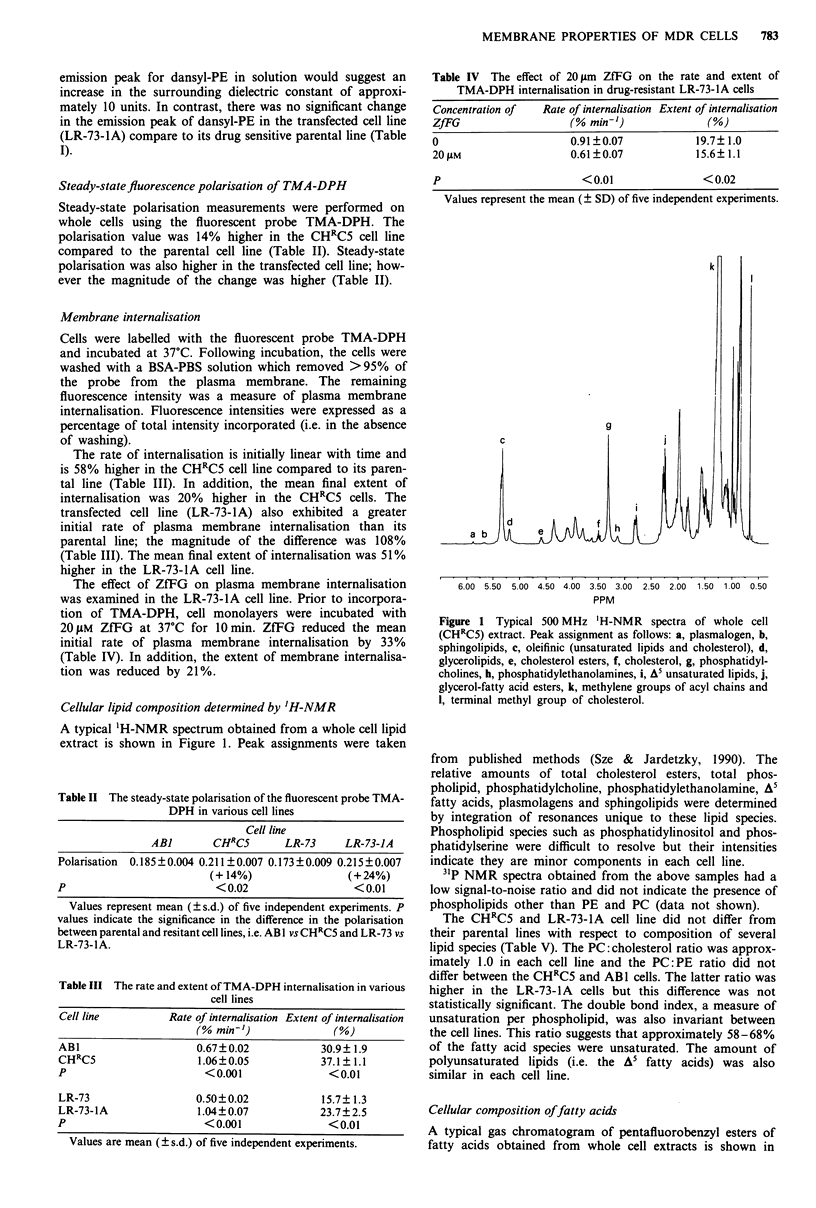
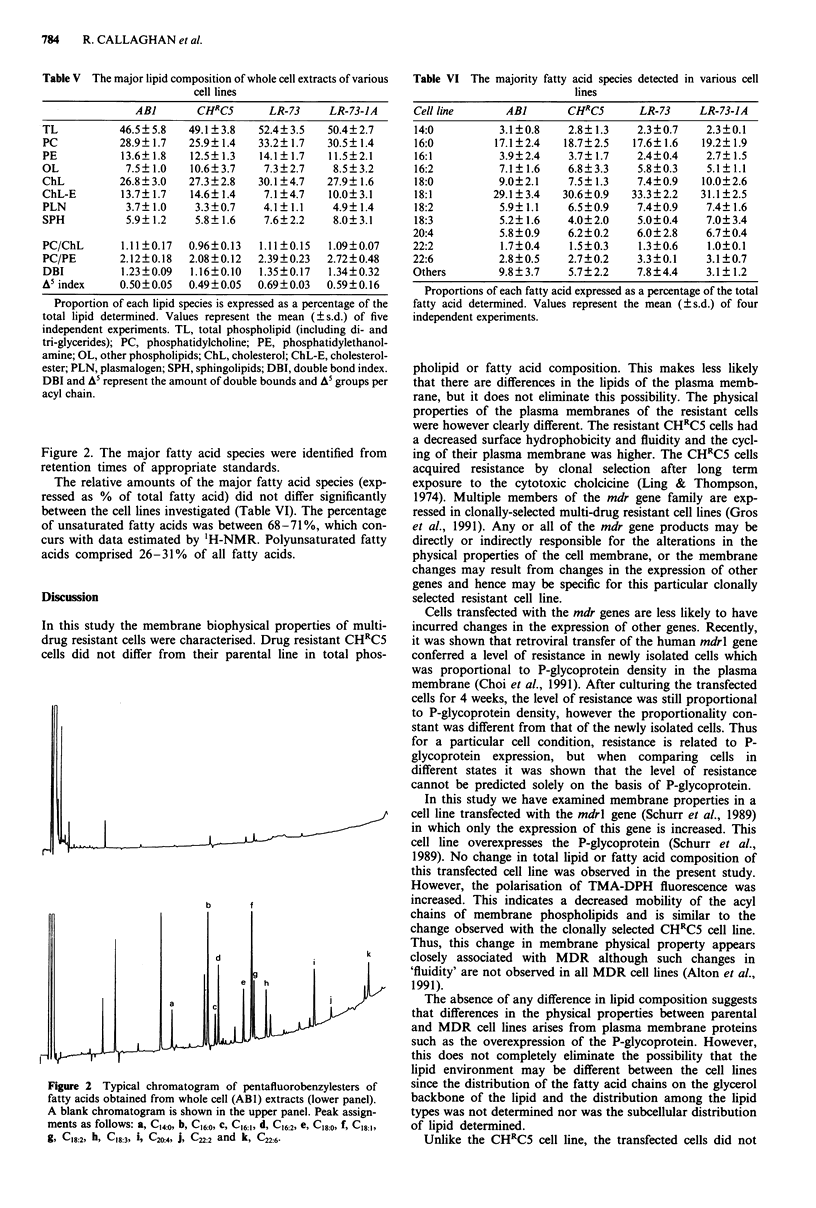
Cell lines selected (CHRC5) and transfected (LR-73-1A) for multi-drug resistance have total lipid compositions which are indistinguishable between resistant and parental cells. Lipid composition was evaluated by 1H NMR and the total fatty acid content by GLC. No change in surface hydrophobicity, as measured with the fluorescent probe dansyl-PE, was observed as a result of transfection of CHO cells with the mdr1 gene. However, the selected cell line, CHRC5, showed a decreased surface hydrophobicity. This decreased surface hydrophobicity was indicated by an 8 nm increase in the fluorescence emission of dansyl-PE in the CHRC5 cell line compared with the AB1. Both resistant cell lines showed an increase in the polarisation of the fluorescent probe, TMA-DPH in the plasma membranes corresponding to a 14% and a 24% change in fluorescence polarisation for the selected and transfected cell lines, respectively. This is indicative of reduced mobility of the acyl chains in the resistant cell lines. Both the CHRC5 and the transfected cell lines showed almost a 2-fold increase in the initial rate of membrane cycling. The membrane cycling could be inhibited by a known bilayer stabiliser, the N-carbobenzoxy-D-Phe-L-Phe-Gly. These results indicate that the properties of the plasma membrane from resistant cells are altered compared with their parental cell line.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arsenault A. L., Ling V., Kartner N. Altered plasma membrane ultrastructure in multidrug-resistant cells. Biochim Biophys Acta. 1988 Feb 18;938(2):315–321. doi: 10.1016/0005-2736(88)90169-1. [DOI] [PubMed] [Google Scholar]
- Basrur V. S., Chitnis M. P., Menon R. S. Cell surface alterations in murine leukaemia P388 adriamycin-resistant cells: studies on lectin-induced agglutination and rearrangement of lectin receptors. Oncology. 1985;42(5):328–331. doi: 10.1159/000226055. [DOI] [PubMed] [Google Scholar]
- Beck W. T. The cell biology of multiple drug resistance. Biochem Pharmacol. 1987 Sep 15;36(18):2879–2887. doi: 10.1016/0006-2952(87)90198-5. [DOI] [PubMed] [Google Scholar]
- Benchekroun M. N., Vrignaud P., Montaudon D., Robert J. Alteration of ganglioside composition and metabolism in doxorubicin-resistant rat tumoral cells. Biochim Biophys Acta. 1988 Dec 16;963(3):553–557. doi: 10.1016/0005-2760(88)90325-6. [DOI] [PubMed] [Google Scholar]
- Boscoboinik D., Epand R. M. Increased cellular internalization of amphiphiles in a multidrug-resistant CHO cell line. Biochim Biophys Acta. 1989 Oct 30;1014(1):53–56. doi: 10.1016/0167-4889(89)90239-5. [DOI] [PubMed] [Google Scholar]
- Choi K., Frommel T. O., Stern R. K., Perez C. F., Kriegler M., Tsuruo T., Roninson I. B. Multidrug resistance after retroviral transfer of the human MDR1 gene correlates with P-glycoprotein density in the plasma membrane and is not affected by cytotoxic selection. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7386–7390. doi: 10.1073/pnas.88.16.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epand R. M., Epand R. F., McKenzie R. C. Effects of viral chemotherapeutic agents on membrane properties. Studies of cyclosporin A, benzyloxycarbonyl-D-Phe-L-Phe-Gly and amantadine. J Biol Chem. 1987 Feb 5;262(4):1526–1529. [PubMed] [Google Scholar]
- Epand R. M., Leon B. T. Hexagonal phase forming propensity detected in phospholipid bilayers with fluorescent probes. Biochemistry. 1992 Feb 11;31(5):1550–1554. doi: 10.1021/bi00120a036. [DOI] [PubMed] [Google Scholar]
- Epand R. M. Virus replication inhibitory peptide inhibits the conversion of phospholipid bilayers to the hexagonal phase. Biosci Rep. 1986 Jul;6(7):647–653. doi: 10.1007/BF01114759. [DOI] [PubMed] [Google Scholar]
- Escriba P. V., Ferrer-Montiel A. V., Ferragut J. A., Gonzalez-Ros J. M. Role of membrane lipids in the interaction of daunomycin with plasma membranes from tumor cells: implications in drug-resistance phenomena. Biochemistry. 1990 Aug 7;29(31):7275–7282. doi: 10.1021/bi00483a017. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Resistance to multiple chemotherapeutic agents in human cancer cells. Trends Pharmacol Sci. 1988 Feb;9(2):54–58. doi: 10.1016/0165-6147(88)90117-4. [DOI] [PubMed] [Google Scholar]
- Gros P., Ben Neriah Y. B., Croop J. M., Housman D. E. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986 Oct 23;323(6090):728–731. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- Illinger D., Poindron P., Fonteneau P., Modollel M., Kuhry J. G. Internalization of the lipophilic fluorescent probe trimethylamino-diphenylhexatriene follows the endocytosis and recycling of the plasma membrane in cells. Biochim Biophys Acta. 1990 Nov 30;1030(1):73–81. doi: 10.1016/0005-2736(90)90240-o. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kelsey D. R., Flanagan T. D., Young J., Yeagle P. L. Peptide inhibitors of enveloped virus infection inhibit phospholipid vesicle fusion and Sendai virus fusion with phospholipid vesicles. J Biol Chem. 1990 Jul 25;265(21):12178–12183. [PubMed] [Google Scholar]
- Kessel D. Probing membrane alterations associated with anthracycline resistance using fluorescent dyes. Biochem Pharmacol. 1988 Nov 15;37(22):4253–4256. doi: 10.1016/0006-2952(88)90603-x. [DOI] [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Ohki S., Arnold K. Surface dielectric constant, surface hydrophobicity and membrane fusion. J Membr Biol. 1990 Apr;114(3):195–203. doi: 10.1007/BF01869214. [DOI] [PubMed] [Google Scholar]
- Peterson R. H., Meyers M. B., Spengler B. A., Biedler J. L. Alteration of plasma membrane glycopeptides and gangliosides of Chinese hamster cells accompanying development of resistance to daunorubicin and vincristine. Cancer Res. 1983 Jan;43(1):222–228. [PubMed] [Google Scholar]
- Ramu A., Glaubiger D., Weintraub H. Differences in lipid composition of doxorubicin-sensitive and -resistant P388 cells. Cancer Treat Rep. 1984 Apr;68(4):637–641. [PubMed] [Google Scholar]
- Riordan J. R., Deuchars K., Kartner N., Alon N., Trent J., Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. 1985 Aug 29-Sep 4Nature. 316(6031):817–819. doi: 10.1038/316817a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld J. M., Hammerberg O., Orvidas M. C. Simplified methods for preparation of microbial fatty acids for analysis by gas chromatography with electron-capture detection. J Chromatogr. 1986 May 28;378(1):9–16. doi: 10.1016/s0378-4347(00)80694-5. [DOI] [PubMed] [Google Scholar]
- Schurr E., Raymond M., Bell J. C., Gros P. Characterization of the multidrug resistance protein expressed in cell clones stably transfected with the mouse mdr1 cDNA. Cancer Res. 1989 May 15;49(10):2729–2733. [PubMed] [Google Scholar]
- Sehested M., Bindslev N., Demant E. J., Skovsgaard T., Jensen P. B. Daunorubicin and vincristine binding to plasma membrane vesicles from daunorubicin-resistant and wild type Ehrlich ascites tumor cells. Biochem Pharmacol. 1989 Sep 15;38(18):3017–3027. doi: 10.1016/0006-2952(89)90010-5. [DOI] [PubMed] [Google Scholar]
- Sehested M., Skovsgaard T., van Deurs B., Winther-Nielsen H. Increase in nonspecific adsorptive endocytosis in anthracycline- and vinca alkaloid-resistant Ehrlich ascites tumor cell lines. J Natl Cancer Inst. 1987 Jan;78(1):171–179. doi: 10.1093/jnci/78.1.171. [DOI] [PubMed] [Google Scholar]
- Sehested M., Skovsgaard T., van Deurs B., Winther-Nielsen H. Increased plasma membrane traffic in daunorubicin resistant P388 leukaemic cells. Effect of daunorubicin and verapamil. Br J Cancer. 1987 Dec;56(6):747–751. doi: 10.1038/bjc.1987.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soranzo C., Ingrosso A., Buffa M., Della Torre G., Gambetta R. A., Zunino F. Changes in the three-dimensional organization of LoVo cells associated with resistance to doxorubicin. Cancer Lett. 1989 Nov 15;48(1):37–41. doi: 10.1016/0304-3835(89)90200-0. [DOI] [PubMed] [Google Scholar]
- Spiegel R. J., Magrath I. T., Shutta J. A. Role of cytoplasmic lipids in altering diphenylhexatriene fluorescence polarization in malignant cells. Cancer Res. 1981 Feb;41(2):452–458. [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze D. Y., Jardetzky O. Characterization of lipid composition in stimulated human lymphocytes by 1H-NMR. Biochim Biophys Acta. 1990 Sep 1;1054(2):198–206. doi: 10.1016/0167-4889(90)90241-5. [DOI] [PubMed] [Google Scholar]
- Tapiero H., Mishal Z., Wioland M., Silber A., Fourcade A., Zwingelstein G. Changes in biophysical parameters and in phospholipid composition associated with resistance to doxorubicin. Anticancer Res. 1986 Jul-Aug;6(4):649–652. [PubMed] [Google Scholar]
- Vrignaud P., Robert J. Free fatty acid uptake is increased in doxorubicin-resistant rat glioblastoma cells. Biochim Biophys Acta. 1987 Aug 7;902(1):149–153. doi: 10.1016/0005-2736(87)90146-5. [DOI] [PubMed] [Google Scholar]
- Wheeler C., Rader R., Kessel D. Membrane alterations associated with progressive adriamycin resistance. Biochem Pharmacol. 1982 Aug 15;31(16):2691–2693. doi: 10.1016/0006-2952(82)90723-7. [DOI] [PubMed] [Google Scholar]
- Wilder P. J., Overman D. K., Tenenholz T. C., Gutierrez P. L. Differences in myristic acid synthesis and in metabolic rate for P388 cells resistant to doxorubicin. J Lipid Res. 1990 Nov;31(11):1973–1982. [PubMed] [Google Scholar]
- Wright L. C., Dyne M., Holmes K. T., Mountford C. E. Phospholipid and ether linked phospholipid content alter with cellular resistance to vinblastine. Biochem Biophys Res Commun. 1985 Dec 17;133(2):539–545. doi: 10.1016/0006-291x(85)90940-4. [DOI] [PubMed] [Google Scholar]


