Abstract
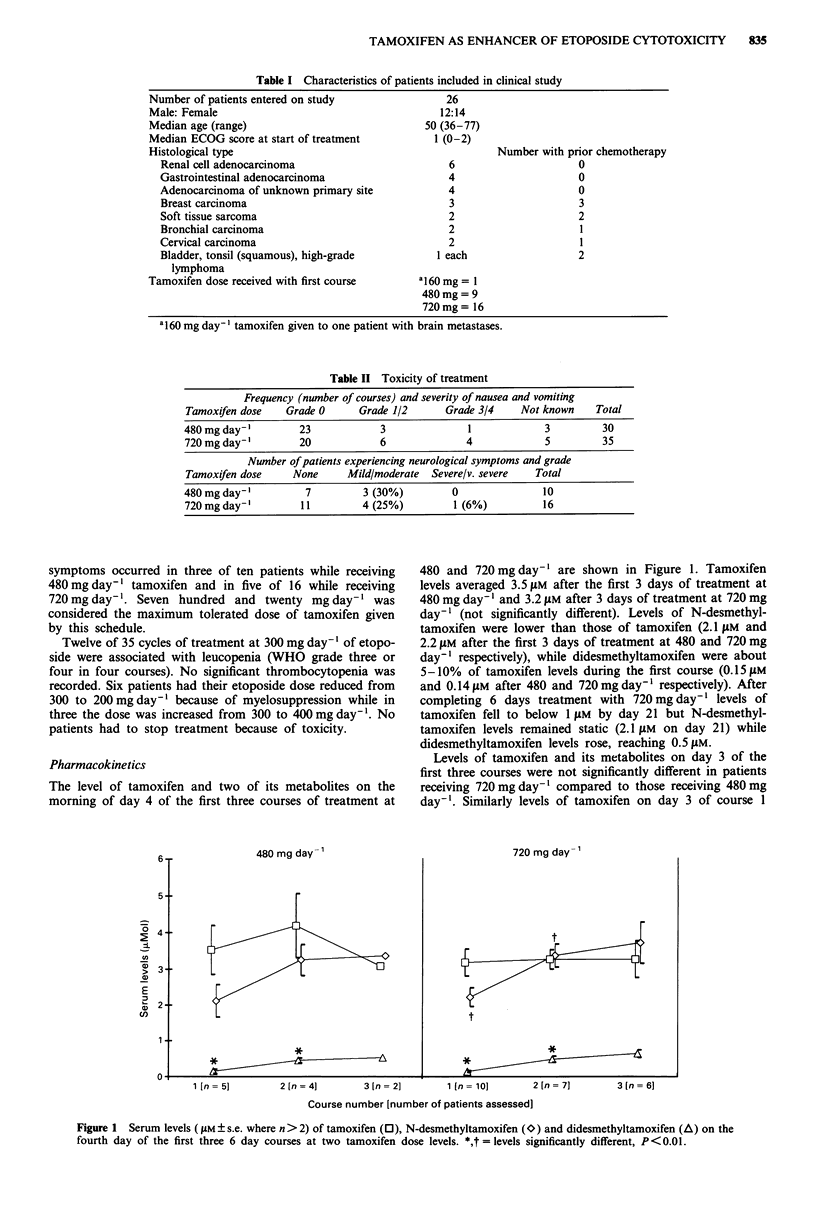
Twenty-six patients with relapsed or drug-resistant cancer were treated with a combination of oral etoposide (300 mg day-1 for 3 days) and high-dose oral tamoxifen as a potential modulator of drug resistance (480 or 720 mg day-1 for 6 days beginning 3 days before etoposide). One patient with relapsed high-grade lymphoma and one with adenocarcinoma of unknown primary site has a partial response. Toxicity consisting of nausea, vomiting and subjective dizziness, unsteadiness of gait and malaise occurred during tamoxifen treatment. Serum levels of tamoxifen averaged 3-3.5 microM on day 4 of all courses of treatment at both 480 and 720 mg day-1. N-desmethyltamoxifen levels were lower than tamoxifen during the first course (2 microM) but increased to equal tamoxifen levels during the second course. Didesmethyltamoxifen levels remained below 1 microM. In vitro, both tamoxifen and the standard modulator of multidrug resistance, verapamil, produced minor enhancement of etoposide cytotoxicity in the MCF-7 wt cell line but produced no enhancement with any other cell line. High, intermittent doses of tamoxifen can be given with acceptable toxicity and produce serum levels that have been shown to modulate drug resistance in vitro. In vitro, however, such levels have no significant effect on etoposide cytotoxicity towards a range of wild-type and MDR cell lines.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam H. K., Patterson J. S., Kemp J. V. Studies on the metabolism and pharmacokinetics of tamoxifen in normal volunteers. Cancer Treat Rep. 1980 Jun-Jul;64(6-7):761–764. [PubMed] [Google Scholar]
- Adam H. K., Patterson J. S., Kemp J. V. Studies on the metabolism and pharmacokinetics of tamoxifen in normal volunteers. Cancer Treat Rep. 1980 Jun-Jul;64(6-7):761–764. [PubMed] [Google Scholar]
- Baas F., Jongsma A. P., Broxterman H. J., Arceci R. J., Housman D., Scheffer G. L., Riethorst A., van Groenigen M., Nieuwint A. W., Joenje H. Non-P-glycoprotein mediated mechanism for multidrug resistance precedes P-glycoprotein expression during in vitro selection for doxorubicin resistance in a human lung cancer cell line. Cancer Res. 1990 Sep 1;50(17):5392–5398. [PubMed] [Google Scholar]
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Beck W. T., Cirtain M. C., Look A. T., Ashmun R. A. Reversal of Vinca alkaloid resistance but not multiple drug resistance in human leukemic cells by verapamil. Cancer Res. 1986 Feb;46(2):778–784. [PubMed] [Google Scholar]
- Berman E., Adams M., Duigou-Osterndorf R., Godfrey L., Clarkson B., Andreeff M. Effect of tamoxifen on cell lines displaying the multidrug-resistant phenotype. Blood. 1991 Feb 15;77(4):818–825. [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Chambers T. C., McAvoy E. M., Jacobs J. W., Eilon G. Protein kinase C phosphorylates P-glycoprotein in multidrug resistant human KB carcinoma cells. J Biol Chem. 1990 May 5;265(13):7679–7686. [PubMed] [Google Scholar]
- Chatterjee M., Harris A. L. Enhancement of Adriamycin cytotoxicity in a multidrug resistant Chinese hamster ovary (CHO) subline, CHO-Adrr, by toremifene and its modulation by alpha 1 acid glycoprotein. Eur J Cancer. 1990 Apr;26(4):432–436. doi: 10.1016/0277-5379(90)90011-h. [DOI] [PubMed] [Google Scholar]
- Chatterjee M., Harris A. L. Reversal of acquired resistance to adriamycin in CHO cells by tamoxifen and 4-hydroxy tamoxifen: role of drug interaction with alpha 1 acid glycoprotein. Br J Cancer. 1990 Nov;62(5):712–717. doi: 10.1038/bjc.1990.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- Dalton W. S., Grogan T. M., Meltzer P. S., Scheper R. J., Durie B. G., Taylor C. W., Miller T. P., Salmon S. E. Drug-resistance in multiple myeloma and non-Hodgkin's lymphoma: detection of P-glycoprotein and potential circumvention by addition of verapamil to chemotherapy. J Clin Oncol. 1989 Apr;7(4):415–424. doi: 10.1200/JCO.1989.7.4.415. [DOI] [PubMed] [Google Scholar]
- DeGregorio M. W., Ford J. M., Benz C. C., Wiebe V. J. Toremifene: pharmacologic and pharmacokinetic basis of reversing multidrug resistance. J Clin Oncol. 1989 Sep;7(9):1359–1364. doi: 10.1200/JCO.1989.7.9.1359. [DOI] [PubMed] [Google Scholar]
- DeVore R. F., Corbett A. H., Osheroff N. Phosphorylation of topoisomerase II by casein kinase II and protein kinase C: effects on enzyme-mediated DNA cleavage/religation and sensitivity to the antineoplastic drugs etoposide and 4'-(9-acridinylamino)methane-sulfon-m-anisidide. Cancer Res. 1992 Apr 15;52(8):2156–2161. [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Ford J. M., Hait W. N. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev. 1990 Sep;42(3):155–199. [PubMed] [Google Scholar]
- Ford J. M., Prozialeck W. C., Hait W. N. Structural features determining activity of phenothiazines and related drugs for inhibition of cell growth and reversal of multidrug resistance. Mol Pharmacol. 1989 Jan;35(1):105–115. [PubMed] [Google Scholar]
- Foster B. J., Grotzinger K. R., McKoy W. M., Rubinstein L. V., Hamilton T. C. Modulation of induced resistance to adriamycin in two human breast cancer cell lines with tamoxifen or perhexiline maleate. Cancer Chemother Pharmacol. 1988;22(2):147–152. doi: 10.1007/BF00257313. [DOI] [PubMed] [Google Scholar]
- Giaccone G., Gazdar A. F., Beck H., Zunino F., Capranico G. Multidrug sensitivity phenotype of human lung cancer cells associated with topoisomerase II expression. Cancer Res. 1992 Apr 1;52(7):1666–1674. [PubMed] [Google Scholar]
- Hindenburg A. A., Baker M. A., Gleyzer E., Stewart V. J., Case N., Taub R. N. Effect of verapamil and other agents on the distribution of anthracyclines and on reversal of drug resistance. Cancer Res. 1987 Mar 1;47(5):1421–1425. [PubMed] [Google Scholar]
- Horgan K., Cooke E., Hallett M. B., Mansel R. E. Inhibition of protein kinase C mediated signal transduction by tamoxifen. Importance for antitumour activity. Biochem Pharmacol. 1986 Dec 15;35(24):4463–4465. doi: 10.1016/0006-2952(86)90764-1. [DOI] [PubMed] [Google Scholar]
- Johnson R. E., Canellos G. P., Young R. C., Chabner B. A., DeVita V. T. Chemotherapy (cyclophosphamide, vincristine, and prednisone) versus radiotherapy (total body irradiation) for stage III-IV poorly differentiated lymphocytic lymphoma. Cancer Treat Rep. 1978 Mar;62(3):321–325. [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kartner N., Riordan J. R., Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983 Sep 23;221(4617):1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- Kaye S. B. Reversal of multidrug resistance. Cancer Treat Rev. 1990 Dec;17 (Suppl A):37–43. doi: 10.1016/0305-7372(90)90014-7. [DOI] [PubMed] [Google Scholar]
- Kessel D. Interactions among membrane transport systems: anthracyclines, calcium antagonists and anti-estrogens. Biochem Pharmacol. 1986 Aug 15;35(16):2825–2826. doi: 10.1016/0006-2952(86)90196-6. [DOI] [PubMed] [Google Scholar]
- Kim R., Hirabayashi N., Nishiyama M., Jinushi K., Toge T., Okada K. Factors contributing to adriamycin sensitivity in human xenograft tumors: the relationship between expression of the MDR1, GST-pi and topoisomerase II genes and tumor sensitivity to adriamycin. Anticancer Res. 1992 Jan-Feb;12(1):241–245. [PubMed] [Google Scholar]
- Lien E. A., Solheim E., Kvinnsland S., Ueland P. M. Identification of 4-hydroxy-N-desmethyltamoxifen as a metabolite of tamoxifen in human bile. Cancer Res. 1988 Apr 15;48(8):2304–2308. [PubMed] [Google Scholar]
- Lien E. A., Solheim E., Lea O. A., Lundgren S., Kvinnsland S., Ueland P. M. Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res. 1989 Apr 15;49(8):2175–2183. [PubMed] [Google Scholar]
- Lien E. A., Ueland P. M., Solheim E., Kvinnsland S. Determination of tamoxifen and four metabolites in serum by low-dispersion liquid chromatography. Clin Chem. 1987 Sep;33(9):1608–1614. [PubMed] [Google Scholar]
- Lien E. A., Wester K., Lønning P. E., Solheim E., Ueland P. M. Distribution of tamoxifen and metabolites into brain tissue and brain metastases in breast cancer patients. Br J Cancer. 1991 Apr;63(4):641–645. doi: 10.1038/bjc.1991.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincke C. R., van der Bliek A. M., Schuurhuis G. J., van der Velde-Koerts T., Smit J. J., Borst P. Multidrug resistance phenotype of human BRO melanoma cells transfected with a wild-type human mdr1 complementary DNA. Cancer Res. 1990 Mar 15;50(6):1779–1785. [PubMed] [Google Scholar]
- Ma L. D., Marquardt D., Takemoto L., Center M. S. Analysis of P-glycoprotein phosphorylation in HL60 cells isolated for resistance to vincristine. J Biol Chem. 1991 Mar 25;266(9):5593–5599. [PubMed] [Google Scholar]
- Merry S., Hamilton T. G., Flanigan P., Freshney R. I., Kaye S. B. Circumvention of pleiotropic drug resistance in subcutaneous tumours in vivo with verapamil and clomipramine. Eur J Cancer. 1991;27(1):31–34. doi: 10.1016/0277-5379(91)90054-h. [DOI] [PubMed] [Google Scholar]
- Millward M. J., Cantwell B. M., Lien E. A., Carmichael J., Harris A. L. Intermittent high-dose tamoxifen as a potential modifier of multidrug resistance. Eur J Cancer. 1992;28A(4-5):805–810. doi: 10.1016/0959-8049(92)90119-m. [DOI] [PubMed] [Google Scholar]
- Nooter K., Oostrum R., Jonker R., van Dekken H., Stokdijk W., van den Engh G. Effect of cyclosporin A on daunorubicin accumulation in multidrug-resistant P388 leukemia cells measured by real-time flow cytometry. Cancer Chemother Pharmacol. 1989;23(5):296–300. doi: 10.1007/BF00292407. [DOI] [PubMed] [Google Scholar]
- Ozols R. F., Cunnion R. E., Klecker R. W., Jr, Hamilton T. C., Ostchega Y., Parrillo J. E., Young R. C. Verapamil and adriamycin in the treatment of drug-resistant ovarian cancer patients. J Clin Oncol. 1987 Apr;5(4):641–647. doi: 10.1200/JCO.1987.5.4.641. [DOI] [PubMed] [Google Scholar]
- Plumb J. A., Milroy R., Kaye S. B. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989 Aug 15;49(16):4435–4440. [PubMed] [Google Scholar]
- Politi P. M., Arnold S. T., Felsted R. L., Sinha B. K. P-glycoprotein-independent mechanism of resistance to VP-16 in multidrug-resistant tumor cell lines: pharmacokinetic and photoaffinity labeling studies. Mol Pharmacol. 1990 Jun;37(6):790–796. [PubMed] [Google Scholar]
- Politi P. M., Sinha B. K. Role of differential drug uptake, efflux, and binding of etoposide in sensitive and resistant human tumor cell lines: implications for the mechanisms of drug resistance. Mol Pharmacol. 1989 Mar;35(3):271–278. [PubMed] [Google Scholar]
- Ramu A., Glaubiger D., Fuks Z. Reversal of acquired resistance to doxorubicin in P388 murine leukemia cells by tamoxifen and other triparanol analogues. Cancer Res. 1984 Oct;44(10):4392–4395. [PubMed] [Google Scholar]
- Riordan J. R., Ling V. Genetic and biochemical characterization of multidrug resistance. Pharmacol Ther. 1985;28(1):51–75. doi: 10.1016/0163-7258(85)90082-8. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Parker C. J., Jordan V. C. Preclinical studies with toremifene as an antitumor agent. Breast Cancer Res Treat. 1990 Aug;16 (Suppl):S9–17. doi: 10.1007/BF01807139. [DOI] [PubMed] [Google Scholar]
- Skovsgaard T. Mechanisms of resistance to daunorubicin in Ehrlich ascites tumor cells. Cancer Res. 1978 Jun;38(6):1785–1791. [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981 May;41(5):1967–1972. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Potentiation of vincristine and Adriamycin effects in human hemopoietic tumor cell lines by calcium antagonists and calmodulin inhibitors. Cancer Res. 1983 May;43(5):2267–2272. [PubMed] [Google Scholar]


