Abstract
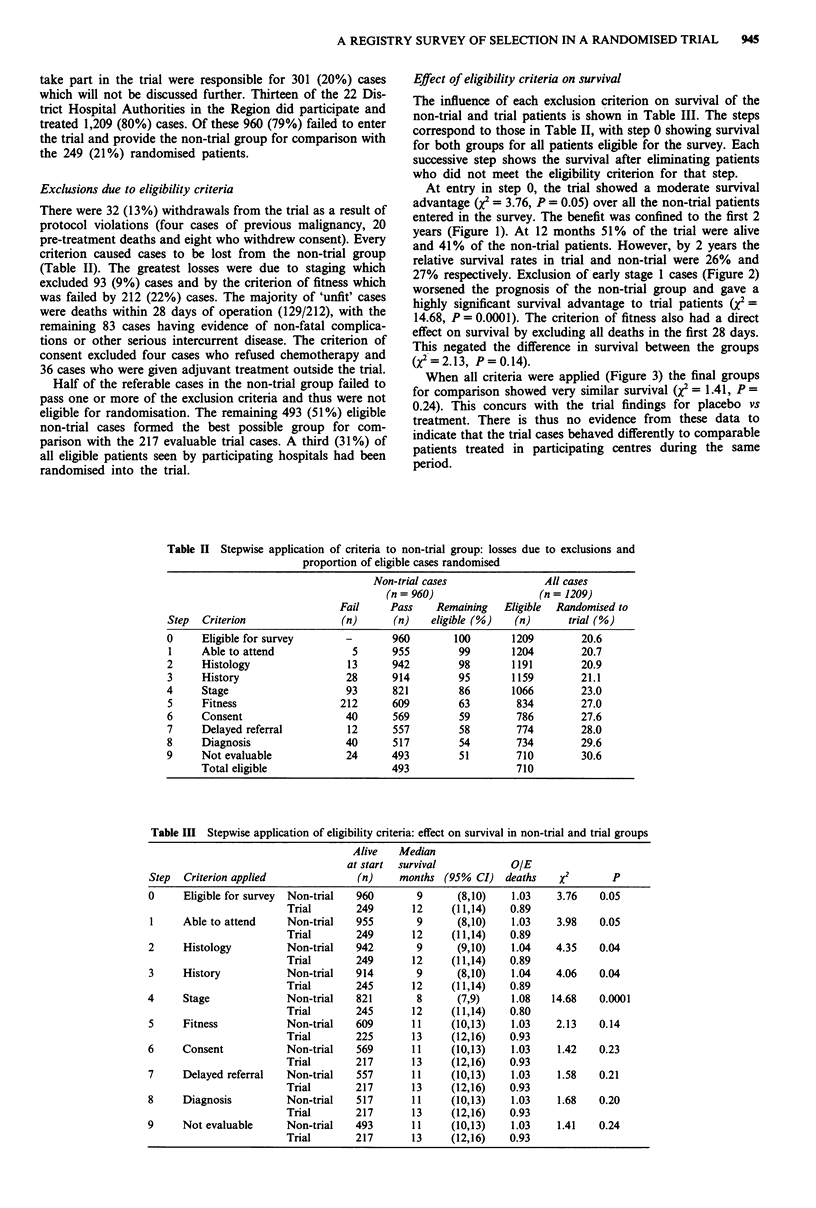
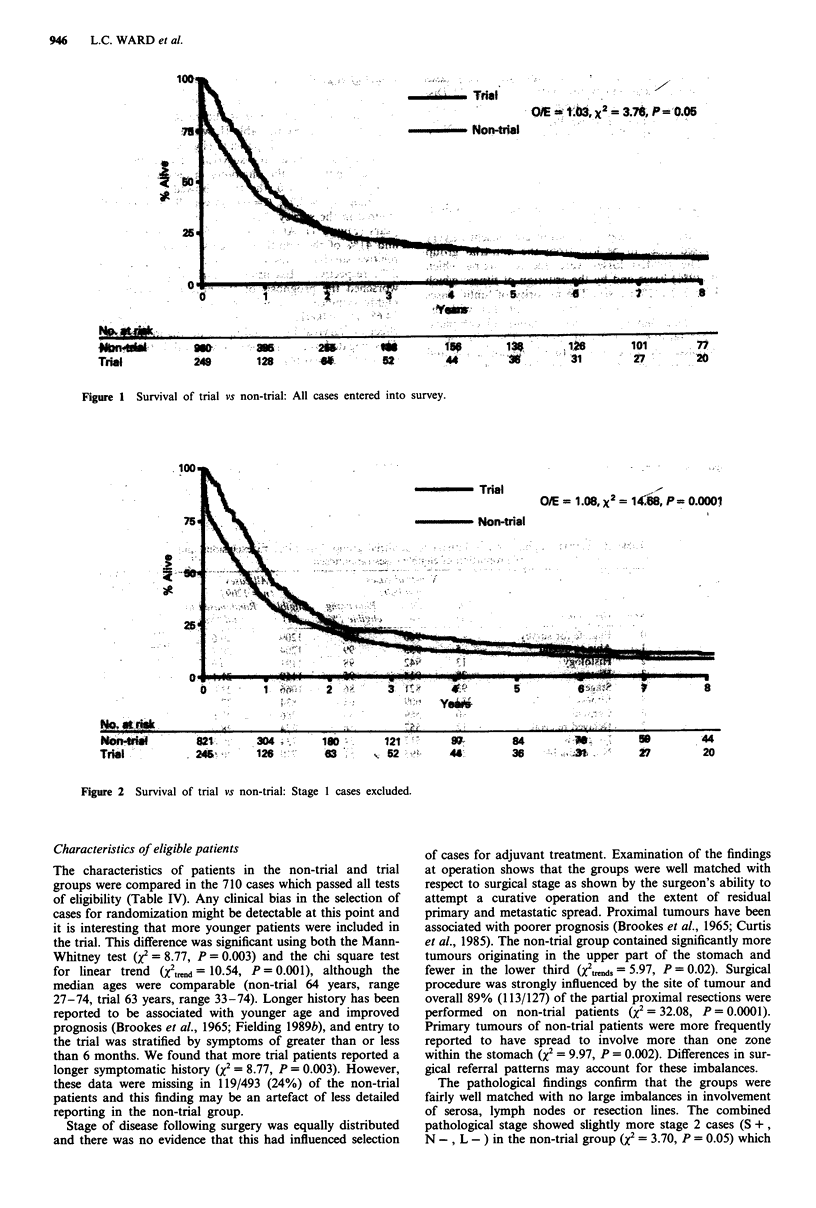
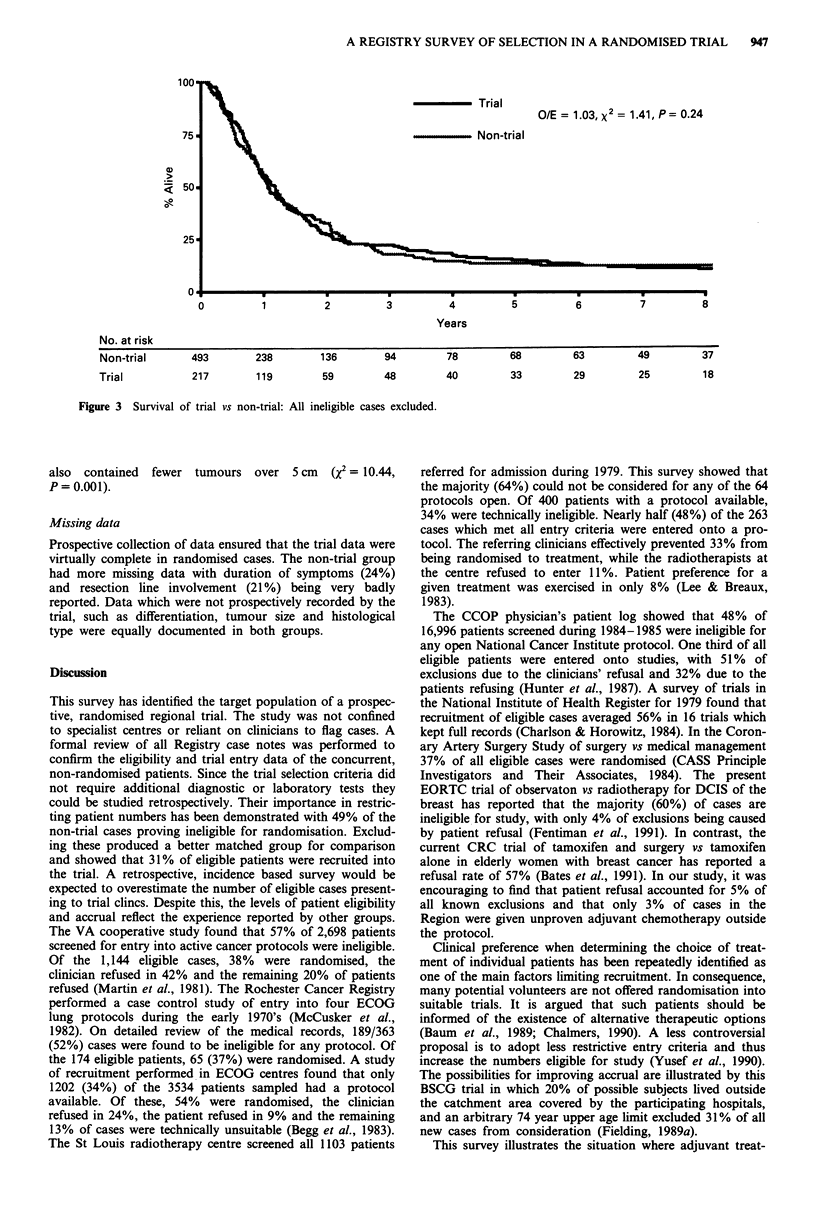
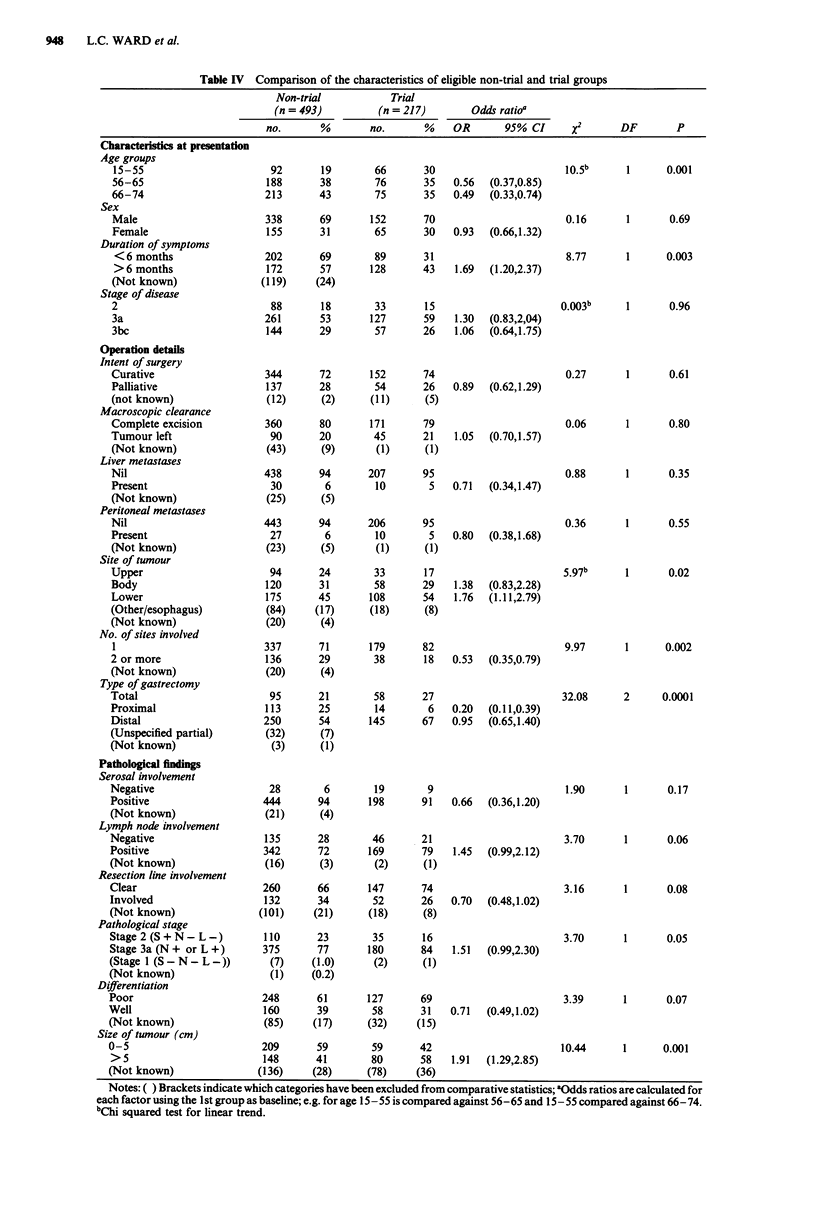
A randomised trial of adjuvant chemotherapy vs placebo in operable stomach cancer recruited 249 patients from the West Midlands Region between 1976-1980. A Cancer Registry survey identified a further 1261 suitable concurrent cases. Trial patients were compared with the 960 non-trial cases from participating Districts. Only 493 (51%) non-trial cases passed all of the prospective trial selection criteria for entry. Stage and fitness caused the majority of exclusions and were also highly prognostic. A univariate analysis comparing eligible patients with the trial showed the two groups to be balanced for the significant independent prognostic factors of the trial. However, differences in patient age and the surgery performed indicate that recruitment may have been influenced by unknown selection factors. This survey highlights the difficulty of retrospective selection and confirms the need for randomised controls. Data available from specialist Registries may be used to help develop new protocols and to verify and extend trial results.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allum W. H., Hallissey M. T., Kelly K. A. Adjuvant chemotherapy in operable gastric cancer. 5 year follow-up of first British Stomach Cancer Group trial. Lancet. 1989 Mar 18;1(8638):571–574. doi: 10.1016/s0140-6736(89)91607-3. [DOI] [PubMed] [Google Scholar]
- Allum W. H., Powell D. J., McConkey C. C., Fielding J. W. Gastric cancer: a 25-year review. Br J Surg. 1989 Jun;76(6):535–540. doi: 10.1002/bjs.1800760604. [DOI] [PubMed] [Google Scholar]
- BROOKES V. S., WATERHOUSE J. A., POWELL D. J. CARCINOMA OF THE STOMACH: A 10-YEAR SURVEY OF RESULTS AND OF FACTORS AFFECTING PROGNOSIS. Br Med J. 1965 Jun 19;1(5450):1577–1583. doi: 10.1136/bmj.1.5450.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshawe K. D., Begent R. H., Newlands E. S., Rustin G. J. What sort of oncology team should treat testicular teratoma? Lancet. 1985 Apr 20;1(8434):930–930. doi: 10.1016/s0140-6736(85)91704-0. [DOI] [PubMed] [Google Scholar]
- Bates T., Riley D. L., Houghton J., Fallowfield L., Baum M. Breast cancer in elderly women: a Cancer Research Campaign trial comparing treatment with tamoxifen and optimal surgery with tamoxifen alone. The Elderly Breast Cancer Working Party. Br J Surg. 1991 May;78(5):591–594. doi: 10.1002/bjs.1800780523. [DOI] [PubMed] [Google Scholar]
- Baum M., Zilkha K., Houghton J. Ethics of clinical research: lessons for the future. BMJ. 1989 Jul 22;299(6693):251–253. doi: 10.1136/bmj.299.6693.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C. B., Engstrom P. F. Eligibility and extrapolation in cancer clinical trials. J Clin Oncol. 1987 Jun;5(6):962–968. doi: 10.1200/JCO.1987.5.6.962. [DOI] [PubMed] [Google Scholar]
- Begg C. B., Zelen M., Carbone P. P., McFadden E. T., Brodovsky H., Engstrom P., Hatfield A., Ingle J., Schwartz B., Stolbach L. Cooperative groups and community hospitals. Measurement of impact in the community hospitals. Cancer. 1983 Nov 1;52(9):1760–1767. doi: 10.1002/1097-0142(19831101)52:9<1760::aid-cncr2820520934>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Byar D. P. The use of data bases and historical controls in treatment comparisons. Recent Results Cancer Res. 1988;111:95–98. doi: 10.1007/978-3-642-83419-6_12. [DOI] [PubMed] [Google Scholar]
- Byar D. P. Why data bases should not replace randomized clinical trials. Biometrics. 1980 Jun;36(2):337–342. [PubMed] [Google Scholar]
- Califf R. M., Pryor D. B., Greenfield J. C., Jr Beyond randomized clinical trials: applying clinical experience in the treatment of patients with coronary artery disease. Circulation. 1986 Dec;74(6):1191–1194. doi: 10.1161/01.cir.74.6.1191. [DOI] [PubMed] [Google Scholar]
- Chalmers T. C. Ethical implications of rejecting patients for clinical trials. JAMA. 1990 Feb 9;263(6):865–865. [PubMed] [Google Scholar]
- Charlson M. E., Horwitz R. I. Applying results of randomised trials to clinical practice: impact of losses before randomisation. Br Med J (Clin Res Ed) 1984 Nov 10;289(6454):1281–1284. doi: 10.1136/bmj.289.6454.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R. E., Kennedy B. J., Myers M. H., Hankey B. F. Evaluation of AJC stomach cancer staging using the SEER population. Semin Oncol. 1985 Mar;12(1):21–31. [PubMed] [Google Scholar]
- Davis K. The comprehensive cohort study: the use of registry data to confirm and extend a randomized trial. Recent Results Cancer Res. 1988;111:138–148. doi: 10.1007/978-3-642-83419-6_17. [DOI] [PubMed] [Google Scholar]
- Elwood P. C. Randomised controlled trials: sampling. Br J Clin Pharmacol. 1982 May;13(5):631–636. doi: 10.1111/j.1365-2125.1982.tb01429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentiman I. S., Julien J. P., van Dongen J. A., van Geel B., Chetty U., Coibion M. Reasons for non-entry of patients with DCIS of the breast into a randomised trial (EORTC 10853). Eur J Cancer. 1991;27(4):450–452. [PubMed] [Google Scholar]
- Fielding J. W., Fagg S. L., Jones B. G., Ellis D., Hockey M. S., Minawa A., Brookes V. S., Craven J. L., Mason M. C., Timothy A. An interim report of a prospective, randomized, controlled study of adjuvant chemotherapy in operable gastric cancer: British Stomach Cancer Group. World J Surg. 1983 May;7(3):390–399. doi: 10.1007/BF01658089. [DOI] [PubMed] [Google Scholar]
- Gehan E. A., Freireich E. J. Non-randomized controls in cancer clinical trials. N Engl J Med. 1974 Jan 24;290(4):198–203. doi: 10.1056/NEJM197401242900405. [DOI] [PubMed] [Google Scholar]
- Green S. B., Byar D. P. Using observational data from registries to compare treatments: the fallacy of omnimetrics. Stat Med. 1984 Oct-Dec;3(4):361–373. doi: 10.1002/sim.4780030413. [DOI] [PubMed] [Google Scholar]
- Hunter C. P., Frelick R. W., Feldman A. R., Bavier A. R., Dunlap W. H., Ford L., Henson D., Macfarlane D., Smart C. R., Yancik R. Selection factors in clinical trials: results from the Community Clinical Oncology Program Physician's Patient Log. Cancer Treat Rep. 1987 Jun;71(6):559–565. [PubMed] [Google Scholar]
- Karjalainen S. Geographical variation in cancer patient survival in Finland: chance, confounding, or effect of treatment? J Epidemiol Community Health. 1990 Sep;44(3):210–214. doi: 10.1136/jech.44.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karjalainen S., Palva I. Do treatment protocols improve end results? A study of survival of patients with multiple myeloma in Finland. BMJ. 1989 Oct 28;299(6707):1069–1072. doi: 10.1136/bmj.299.6707.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Breaux S. R. Accrual of radiotherapy patients to clinical trials. Cancer. 1983 Sep 15;52(6):1014–1016. doi: 10.1002/1097-0142(19830915)52:6<1014::aid-cncr2820520614>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Mantel N. Cautions on the use of medical databases. Stat Med. 1983 Jul-Sep;2(3):355–362. doi: 10.1002/sim.4780020307. [DOI] [PubMed] [Google Scholar]
- Matthews H. R., Powell D. J., McConkey C. C. Effect of surgical experience on the results of resection for oesophageal carcinoma. Br J Surg. 1986 Aug;73(8):621–623. doi: 10.1002/bjs.1800730811. [DOI] [PubMed] [Google Scholar]
- McCusker J., Wax A., Bennett J. M. Cancer patient accessions into clinical trials: a pilot investigation into some patient and physician determinants of entry. Am J Clin Oncol. 1982 Apr;5(2):227–236. doi: 10.1097/00000421-198204000-00072. [DOI] [PubMed] [Google Scholar]
- McDonald C. J., Hui S. L. The analysis of humongous databases: problems and promises. Stat Med. 1991 Apr;10(4):511–518. doi: 10.1002/sim.4780100404. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock S. J. The combination of randomized and historical controls in clinical trials. J Chronic Dis. 1976 Mar;29(3):175–188. doi: 10.1016/0021-9681(76)90044-8. [DOI] [PubMed] [Google Scholar]
- Stiller C. A. Survival of patients with cancer. BMJ. 1989 Oct 28;299(6707):1058–1059. doi: 10.1136/bmj.299.6707.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate H. C., Rawlinson J. B., Freedman L. S. Randomised comparative studies in the treatment of cancer in the United Kingdom: room for improvement? Lancet. 1979 Sep 22;2(8143):623–625. doi: 10.1016/s0140-6736(79)91676-3. [DOI] [PubMed] [Google Scholar]
- Yusuf S., Held P., Teo K. K., Toretsky E. R. Selection of patients for randomized controlled trials: implications of wide or narrow eligibility criteria. Stat Med. 1990 Jan-Feb;9(1-2):73–86. doi: 10.1002/sim.4780090114. [DOI] [PubMed] [Google Scholar]



