Abstract
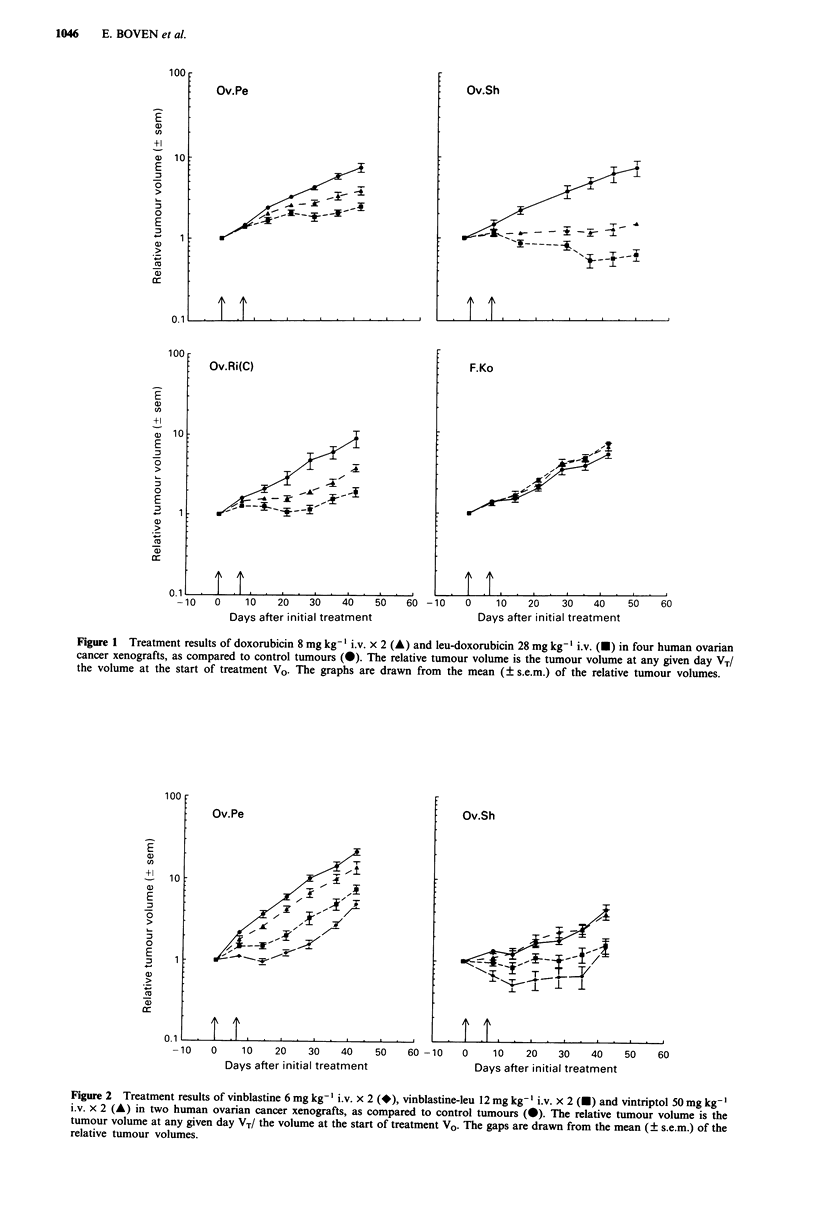
N-l-leucyl-doxorubicin and vinblastine-isoleucinate can be considered as relatively non-toxic prodrugs from doxorubicin and vinblastine, respectively. A comparative analysis was carried out of the anti-tumour activity of the four compounds as well as vintriptol in four human ovarian cancer xenografts different in histology, growth rate and chemosensitivity. Injections were given i.v. weekly twice into mice bearing well-established s.c. tumours. At equitoxic doses, the amount of drug administered for N-l-leucyl-doxorubicin and vinblastine-isoleucinate was respectively 3-fold and 2-fold higher than the doses of the parent compound. N-l-leucyl-doxorubicin induced a growth inhibition > 50% in three out of four human ovarian cancer lines. The anti-tumour effects obtained were significantly better (P < 0.01) than in the case of doxorubicin. Vinblastine-isoleucinate studied in two of these lines could induce a growth inhibition of > 50%. This prodrug appeared slightly less effective than vinblastine. Insignificant growth inhibition (< 50%) was obtained by vintriptol.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baurain R., Masquelier M., Deprez-De Campeneere D., Trouet A. Amino acid and dipeptide derivatives of daunorubicin. 2. Cellular pharmacology and antitumor activity on L1210 leukemic cells in vitro and in vivo. J Med Chem. 1980 Nov;23(11):1171–1174. doi: 10.1021/jm00185a004. [DOI] [PubMed] [Google Scholar]
- Bhushana Rao K. S., Collard M. P., Dejonghe J. P., Atassi G., Hannart J. A., Trouet A. Vinblastin-23-oyl amino acid derivatives: chemistry, physicochemical data, toxicity, and antitumor activities against P388 and L1210 leukemias. J Med Chem. 1985 Aug;28(8):1079–1088. doi: 10.1021/jm00146a017. [DOI] [PubMed] [Google Scholar]
- Boven E., Nauta M. M., Schluper H. M., Elferink F., van der Vijgh W. J., Pinedo H. M. Secondary screening of platinum compounds in human ovarian cancer xenografts in nude mice. Eur J Cancer Clin Oncol. 1985 Oct;21(10):1253–1260. doi: 10.1016/0277-5379(85)90023-9. [DOI] [PubMed] [Google Scholar]
- Boven E., Schlüper H. M., Erkelens C. A., Pinedo H. M. Doxorubicin compared with related compounds in a nude mouse model for human ovarian cancer. Eur J Cancer. 1990;26(9):983–986. doi: 10.1016/0277-5379(90)90626-5. [DOI] [PubMed] [Google Scholar]
- Boven E., Winograd B., Fodstad O., Lobbezoo M. W., Pinedo H. M. Preclinical phase II studies in human tumor lines: a European multicenter study. Eur J Cancer Clin Oncol. 1988 Mar;24(3):567–573. doi: 10.1016/s0277-5379(98)90039-6. [DOI] [PubMed] [Google Scholar]
- Boven E., van der Vijgh W. J., Nauta M. M., Schlüper H. M., Pinedo H. M. Comparative activity and distribution studies of five platinum analogues in nude mice bearing human ovarian carcinoma xenografts. Cancer Res. 1985 Jan;45(1):86–90. [PubMed] [Google Scholar]
- Deprez-de Campeneere D., Baurain R., Trouet A. Accumulation and metabolism of new anthracycline derivatives in the heart after IV injection into mice. Cancer Chemother Pharmacol. 1982;8(2):193–197. doi: 10.1007/BF00255483. [DOI] [PubMed] [Google Scholar]
- Hendriks H. R., Langdon S., Berger D. P., Breistøl K., Fiebig H. H., Fodstad O., Schwartsmann G. Comparative antitumour activity of vinblastine-isoleucinate and related vinca alkaloids in human tumour xenografts. Eur J Cancer. 1992;28A(4-5):767–773. doi: 10.1016/0959-8049(92)90112-f. [DOI] [PubMed] [Google Scholar]
- JOHNSON I. S., ARMSTRONG J. G., GORMAN M., BURNETT J. P., Jr THE VINCA ALKALOIDS: A NEW CLASS OF ONCOLYTIC AGENTS. Cancer Res. 1963 Sep;23:1390–1427. [PubMed] [Google Scholar]
- Jaenke R. S., Deprez-DeCampeneere D., Trouet A. Cardiotoxicity and comparative pharmacokinetics of six anthracyclines in the rabbit. Cancer Res. 1980 Oct;40(10):3530–3536. [PubMed] [Google Scholar]
- Maciewicz R. A., Wardale R. J., Etherington D. J., Paraskeva C. Immunodetection of cathepsins B and L present in and secreted from human pre-malignant and malignant colorectal tumour cell lines. Int J Cancer. 1989 Mar 15;43(3):478–486. doi: 10.1002/ijc.2910430323. [DOI] [PubMed] [Google Scholar]
- Masquelier M., Baurain R., Trouet A. Amino acid and dipeptide derivatives of daunorubicin. 1. Synthesis, physicochemical properties, and lysosomal digestion. J Med Chem. 1980 Nov;23(11):1166–1170. doi: 10.1021/jm00185a003. [DOI] [PubMed] [Google Scholar]
- Oosterkamp H. M., Vlasveld L. T., Wanders J., Beijnen J. H., van Tellingen O., Dubbelman A. C., Simonetti G. P., Franklin H. R., Vermorken J. B., Ten Bokkel Huinink W. W. Phase I study of vintriptol, a tryptophan ester of vinblastine. Eur J Cancer. 1991;27(10):1222–1226. doi: 10.1016/0277-5379(91)90085-r. [DOI] [PubMed] [Google Scholar]
- Thorpe S. M., Rochefort H., Garcia M., Freiss G., Christensen I. J., Khalaf S., Paolucci F., Pau B., Rasmussen B. B., Rose C. Association between high concentrations of Mr 52,000 cathepsin D and poor prognosis in primary human breast cancer. Cancer Res. 1989 Nov 1;49(21):6008–6014. [PubMed] [Google Scholar]
- Waller D. G., George C. F. Prodrugs. Br J Clin Pharmacol. 1989 Nov;28(5):497–507. doi: 10.1111/j.1365-2125.1989.tb03535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. C., Ozols R. F., Myers C. E. The anthracycline antineoplastic drugs. N Engl J Med. 1981 Jul 16;305(3):139–153. doi: 10.1056/NEJM198107163050305. [DOI] [PubMed] [Google Scholar]



