Abstract
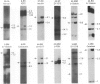
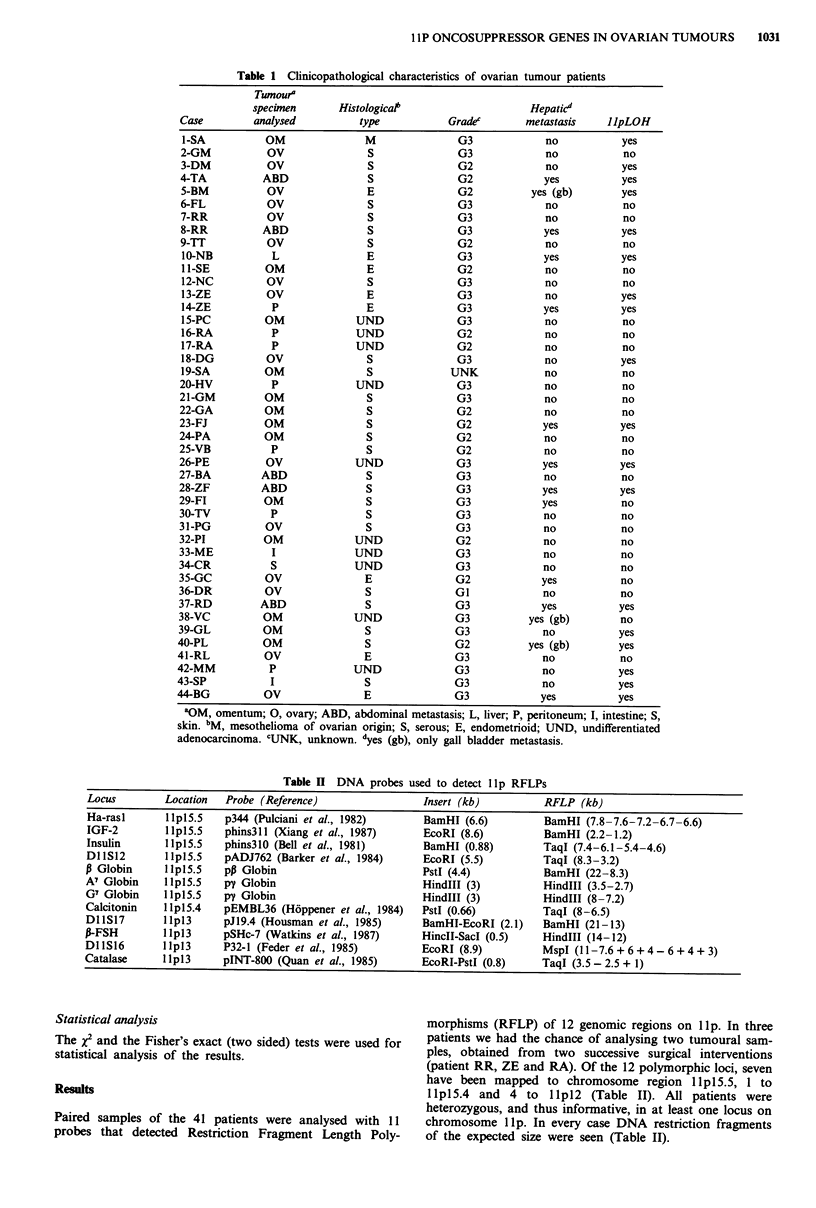
In this study, 44 primary or metastatic human ovarian tumours were tested for allelic deletions on the short arm of chromosome 11. Analysis of 12 polymorphic loci by Southern blotting evidenced loss of heterozygosity (LOH) in at least one locus in 41% of cases. Moreover, two hot spots of deletions were tentatively mapped on 11p13 and 11p15.5. Our results demonstrated that LOH at 11p is a common event in ovarian carcinomas and were indicative of the possible existence in 11p of two oncosuppressor genes involved in ovarian carcinogenesis. The similarity observed with 11p allelic losses in Wilms tumours, clustered in 11p13 and 11p15.5 too, suggests that deletion and possibly inactivation of the same growth regulatory genes (WT genes) could also contribute to development of the malignant phenotype in ovarian carcinomas. Finally, a statistically significant association (P = 0.005) between 11p deletions and hepatic involvement was suggested by the analysis of distribution of 11p LOH relative to different clinical and pathological parameters of the tumour patients.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Lidereau R., Theillet C., Callahan R. Reduction to homozygosity of genes on chromosome 11 in human breast neoplasia. Science. 1987 Oct 9;238(4824):185–188. doi: 10.1126/science.3659909. [DOI] [PubMed] [Google Scholar]
- Barker D., Holm T., White R. A locus on chromosome 11p with multiple restriction site polymorphisms. Am J Hum Genet. 1984 Nov;36(6):1159–1171. [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltz E. M., Kefford R. F., Leary J. A., Houghton C. R., Friedlander M. L. Amplification of c-ras-Ki oncogene in human ovarian tumours. Int J Cancer. 1989 Mar 15;43(3):428–430. doi: 10.1002/ijc.2910430314. [DOI] [PubMed] [Google Scholar]
- Devilee P., van den Broek M., Mannens M., Slater R., Cornelisse C. J., Westerveld A., Khan P. M. Differences in patterns of allelic loss between two common types of adult cancer, breast and colon carcinoma, and Wilms' tumor of childhood. Int J Cancer. 1991 Apr 1;47(6):817–821. doi: 10.1002/ijc.2910470604. [DOI] [PubMed] [Google Scholar]
- Eccles D. M., Cranston G., Steel C. M., Nakamura Y., Leonard R. C. Allele losses on chromosome 17 in human epithelial ovarian carcinoma. Oncogene. 1990 Oct;5(10):1599–1601. [PubMed] [Google Scholar]
- Ehlen T., Dubeau L. Loss of heterozygosity on chromosomal segments 3p, 6q and 11p in human ovarian carcinomas. Oncogene. 1990 Feb;5(2):219–223. [PubMed] [Google Scholar]
- Enomoto T., Weghorst C. M., Inoue M., Tanizawa O., Rice J. M. K-ras activation occurs frequently in mucinous adenocarcinomas and rarely in other common epithelial tumors of the human ovary. Am J Pathol. 1991 Oct;139(4):777–785. [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Feinberg A. P., Hamilton S. H., Vogelstein B. Loss of genes on the short arm of chromosome 11 in bladder cancer. 1985 Nov 28-Dec 4Nature. 318(6044):377–380. doi: 10.1038/318377a0. [DOI] [PubMed] [Google Scholar]
- Feder J., Yen L., Wijsman E., Wang L., Wilkins L., Schroder J., Spurr N., Cann H., Blumenberg M., Cavalli-Sforza L. L. A systematic approach for detecting high-frequency restriction fragment length polymorphisms using large genomic probes. Am J Hum Genet. 1985 Jul;37(4):635–649. [PMC free article] [PubMed] [Google Scholar]
- Gessler M., Poustka A., Cavenee W., Neve R. L., Orkin S. H., Bruns G. A. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990 Feb 22;343(6260):774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- Haber D. A., Buckler A. J., Glaser T., Call K. M., Pelletier J., Sohn R. L., Douglass E. C., Housman D. E. An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms' tumor. Cell. 1990 Jun 29;61(7):1257–1269. doi: 10.1016/0092-8674(90)90690-g. [DOI] [PubMed] [Google Scholar]
- Henry I., Grandjouan S., Couillin P., Barichard F., Huerre-Jeanpierre C., Glaser T., Philip T., Lenoir G., Chaussain J. L., Junien C. Tumor-specific loss of 11p15.5 alleles in del11p13 Wilms tumor and in familial adrenocortical carcinoma. Proc Natl Acad Sci U S A. 1989 May;86(9):3247–3251. doi: 10.1073/pnas.86.9.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höppener J. W., Steenbergh P. H., Zandberg J., Bakker E., Pearson P. L., Geurts van Kessel A. H., Jansz H. S., Lips C. J. Localization of the polymorphic human calcitonin gene on chromosome 11. Hum Genet. 1984;66(4):309–312. doi: 10.1007/BF00287635. [DOI] [PubMed] [Google Scholar]
- Koufos A., Grundy P., Morgan K., Aleck K. A., Hadro T., Lampkin B. C., Kalbakji A., Cavenee W. K. Familial Wiedemann-Beckwith syndrome and a second Wilms tumor locus both map to 11p15.5. Am J Hum Genet. 1989 May;44(5):711–719. [PMC free article] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Copeland N. G., Jenkins N. A., Lampkin B. C., Cavenee W. K. Loss of heterozygosity in three embryonal tumours suggests a common pathogenetic mechanism. Nature. 1985 Jul 25;316(6026):330–334. doi: 10.1038/316330a0. [DOI] [PubMed] [Google Scholar]
- Lee J. H., Kavanagh J. J., Wildrick D. M., Wharton J. T., Blick M. Frequent loss of heterozygosity on chromosomes 6q, 11, and 17 in human ovarian carcinomas. Cancer Res. 1990 May 1;50(9):2724–2728. [PubMed] [Google Scholar]
- Li S. B., Schwartz P. E., Lee W. H., Yang-Feng T. L. Allele loss at the retinoblastoma locus in human ovarian cancer. J Natl Cancer Inst. 1991 May 1;83(9):637–640. doi: 10.1093/jnci/83.9.637. [DOI] [PubMed] [Google Scholar]
- Lothe R. A., Fosså S. D., Stenwig A. E., Nakamura Y., White R., Børresen A. L., Brøgger A. Loss of 3p or 11p alleles is associated with testicular cancer tumors. Genomics. 1989 Jul;5(1):134–138. doi: 10.1016/0888-7543(89)90097-9. [DOI] [PubMed] [Google Scholar]
- Ludwig C. U., Raefle G., Dalquen P., Stulz P., Stahel R., Obrecht J. P. Allelic loss on the short arm of chromosome 11 in non-small-cell lung cancer. Int J Cancer. 1991 Nov 11;49(5):661–665. doi: 10.1002/ijc.2910490506. [DOI] [PubMed] [Google Scholar]
- Mackay J., Elder P. A., Porteous D. J., Steel C. M., Hawkins R. A., Going J. J., Chetty U. Partial deletion of chromosome 11p in breast cancer correlates with size of primary tumour and oestrogen receptor level. Br J Cancer. 1988 Dec;58(6):710–714. doi: 10.1038/bjc.1988.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. J. Tumor suppressor genes. Cell. 1991 Jan 25;64(2):313–326. doi: 10.1016/0092-8674(91)90641-b. [DOI] [PubMed] [Google Scholar]
- Okamoto A., Sameshima Y., Yokoyama S., Terashima Y., Sugimura T., Terada M., Yokota J. Frequent allelic losses and mutations of the p53 gene in human ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5171–5176. [PubMed] [Google Scholar]
- Pelletier J., Bruening W., Kashtan C. E., Mauer S. M., Manivel J. C., Striegel J. E., Houghton D. C., Junien C., Habib R., Fouser L. Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell. 1991 Oct 18;67(2):437–447. doi: 10.1016/0092-8674(91)90194-4. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Bruening W., Li F. P., Haber D. A., Glaser T., Housman D. E. WT1 mutations contribute to abnormal genital system development and hereditary Wilms' tumour. Nature. 1991 Oct 3;353(6343):431–434. doi: 10.1038/353431a0. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Schalling M., Buckler A. J., Rogers A., Haber D. A., Housman D. Expression of the Wilms' tumor gene WT1 in the murine urogenital system. Genes Dev. 1991 Aug;5(8):1345–1356. doi: 10.1101/gad.5.8.1345. [DOI] [PubMed] [Google Scholar]
- Pulciani S., Santos E., Lauver A. V., Long L. K., Barbacid M. Transforming genes in human tumors. J Cell Biochem. 1982;20(1):51–61. doi: 10.1002/jcb.240200106. [DOI] [PubMed] [Google Scholar]
- Quan F., Korneluk R. G., MacLeod H. L., Tsui L. C., Gravel R. A. An RFLP associated with the human catalase gene. Nucleic Acids Res. 1985 Nov 25;13(22):8288–8288. doi: 10.1093/nar/13.22.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve A. E., Sih S. A., Raizis A. M., Feinberg A. P. Loss of allelic heterozygosity at a second locus on chromosome 11 in sporadic Wilms' tumor cells. Mol Cell Biol. 1989 Apr;9(4):1799–1803. doi: 10.1128/mcb.9.4.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose E. A., Glaser T., Jones C., Smith C. L., Lewis W. H., Call K. M., Minden M., Champagne E., Bonetta L., Yeger H. Complete physical map of the WAGR region of 11p13 localizes a candidate Wilms' tumor gene. Cell. 1990 Feb 9;60(3):495–508. doi: 10.1016/0092-8674(90)90600-j. [DOI] [PubMed] [Google Scholar]
- Russell S. E., Hickey G. I., Lowry W. S., White P., Atkinson R. J. Allele loss from chromosome 17 in ovarian cancer. Oncogene. 1990 Oct;5(10):1581–1583. [PubMed] [Google Scholar]
- Sato T., Saito H., Morita R., Koi S., Lee J. H., Nakamura Y. Allelotype of human ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5118–5122. [PubMed] [Google Scholar]
- Scott J., Cowell J., Robertson M. E., Priestley L. M., Wadey R., Hopkins B., Pritchard J., Bell G. I., Rall L. B., Graham C. F. Insulin-like growth factor-II gene expression in Wilms' tumour and embryonic tissues. Nature. 1985 Sep 19;317(6034):260–262. doi: 10.1038/317260a0. [DOI] [PubMed] [Google Scholar]
- Scrable H. J., Witte D. P., Lampkin B. C., Cavenee W. K. Chromosomal localization of the human rhabdomyosarcoma locus by mitotic recombination mapping. Nature. 1987 Oct 15;329(6140):645–647. doi: 10.1038/329645a0. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Viel A., De Pascale L., Toffoli G., Tumiotto L., Miotto E., Boiocchi M. Frequent occurrence of Ha-rasl allelic deletion in human ovarian adenocarcinomas. Tumori. 1991 Feb 28;77(1):16–20. doi: 10.1177/030089169107700104. [DOI] [PubMed] [Google Scholar]
- Viel A., Maestro R., Toffoli G., Grion G., Boiocchi M. c-myc overexpression is a tumor-specific phenomenon in a subset of human colorectal carcinomas. J Cancer Res Clin Oncol. 1990;116(3):288–294. doi: 10.1007/BF01612905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadey R. B., Pal N., Buckle B., Yeomans E., Pritchard J., Cowell J. K. Loss of heterozygosity in Wilms' tumour involves two distinct regions of chromosome 11. Oncogene. 1990 Jun;5(6):901–907. [PubMed] [Google Scholar]
- Watkins P. C., Eddy R., Beck A. K., Vellucci V., Leverone B., Tanzi R. E., Gusella J. F., Shows T. B. DNA sequence and regional assignment of the human follicle-stimulating hormone beta-subunit gene to the short arm of human chromosome 11. DNA. 1987 Jun;6(3):205–212. doi: 10.1089/dna.1987.6.205. [DOI] [PubMed] [Google Scholar]
- Weston A., Willey J. C., Modali R., Sugimura H., McDowell E. M., Resau J., Light B., Haugen A., Mann D. L., Trump B. F. Differential DNA sequence deletions from chromosomes 3, 11, 13, and 17 in squamous-cell carcinoma, large-cell carcinoma, and adenocarcinoma of the human lung. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5099–5103. doi: 10.1073/pnas.86.13.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang K., Karam J. H., Bell G. I. BamHI RFLP at the insulin-like growth factor II (IGF2) locus on chromosome 11. Nucleic Acids Res. 1987 Sep 25;15(18):7655–7655. doi: 10.1093/nar/15.18.7655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J. P., Robinson W. R., Ehlen T., Yu M. C., Dubeau L. Distinction of low grade from high grade human ovarian carcinomas on the basis of losses of heterozygosity on chromosomes 3, 6, and 11 and HER-2/neu gene amplification. Cancer Res. 1991 Aug 1;51(15):4045–4051. [PubMed] [Google Scholar]
- Zhou D. J., Gonzalez-Cadavid N., Ahuja H., Battifora H., Moore G. E., Cline M. J. A unique pattern of proto-oncogene abnormalities in ovarian adenocarcinomas. Cancer. 1988 Oct 15;62(8):1573–1576. doi: 10.1002/1097-0142(19881015)62:8<1573::aid-cncr2820620819>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- van 't Veer L. J., Hermens R., van den Berg-Bakker L. A., Cheng N. C., Fleuren G. J., Bos J. L., Cleton F. J., Schrier P. I. ras oncogene activation in human ovarian carcinoma. Oncogene. 1988 Feb;2(2):157–165. [PubMed] [Google Scholar]