Abstract
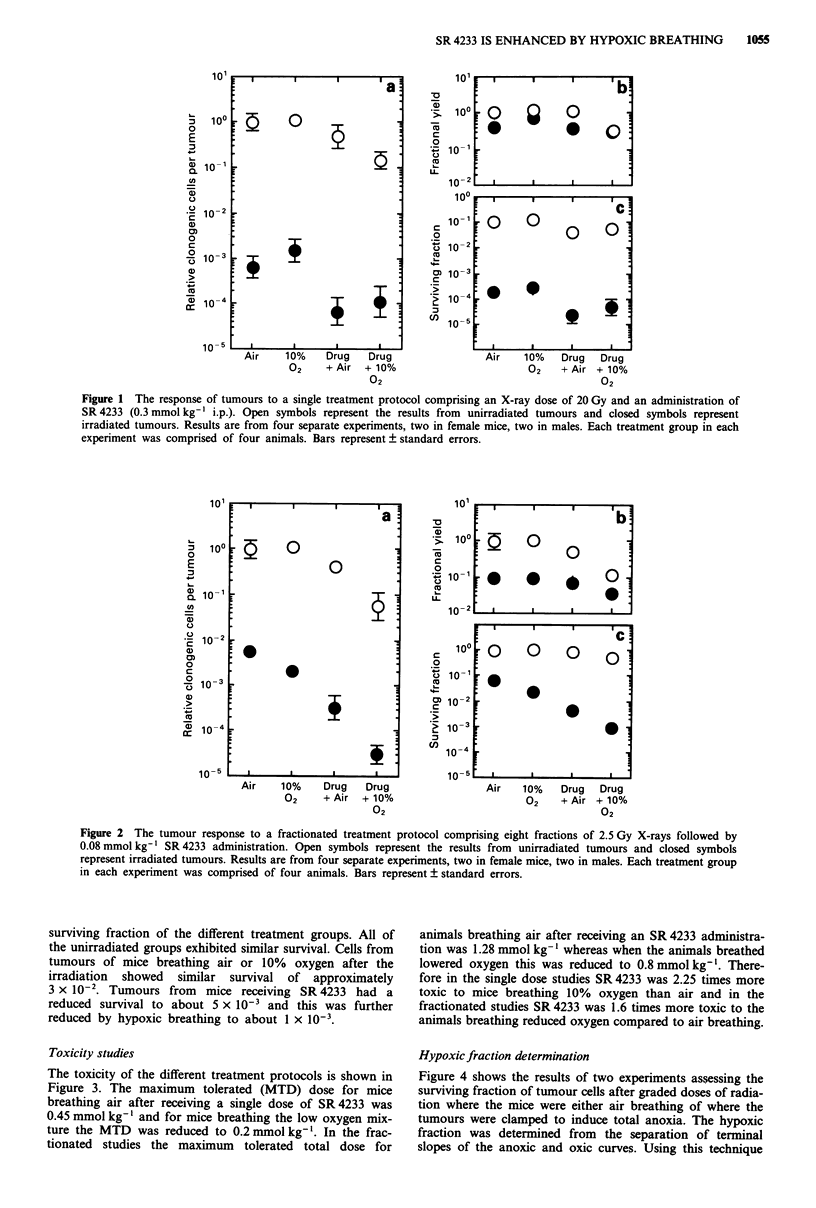
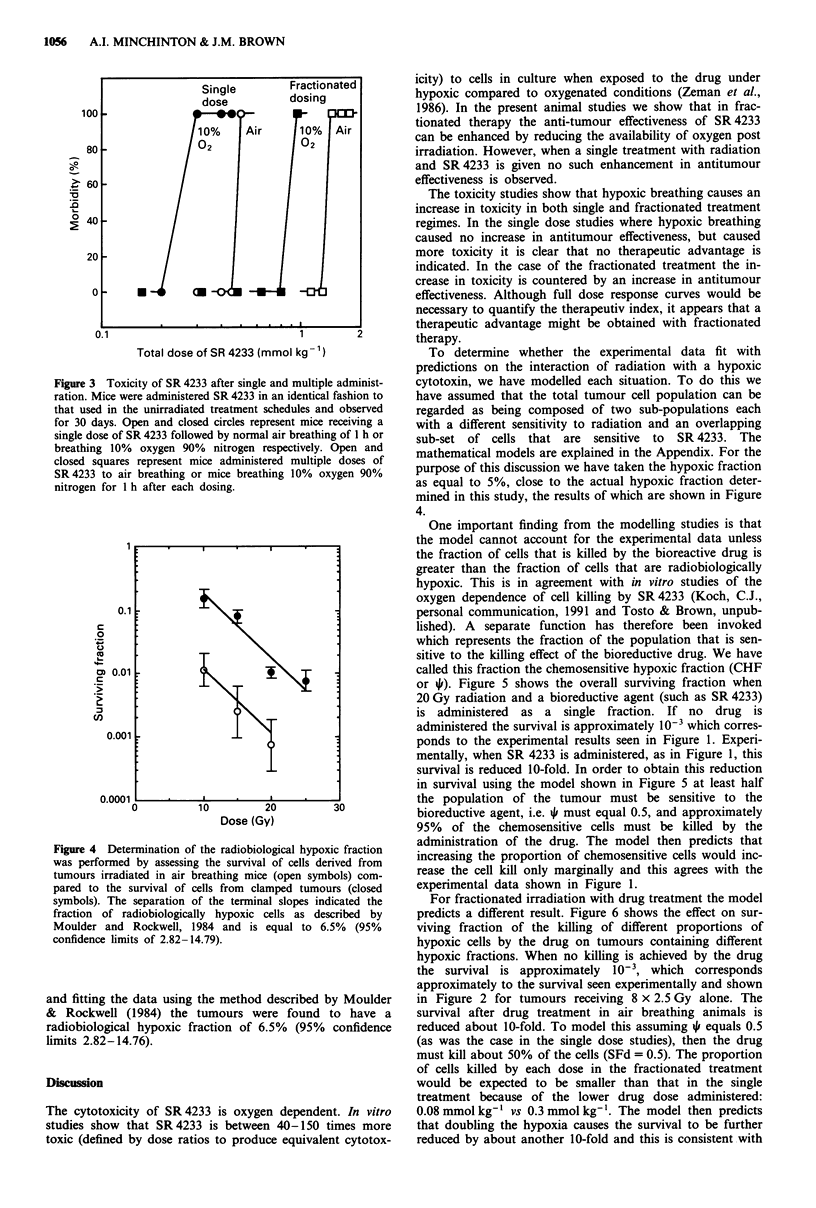
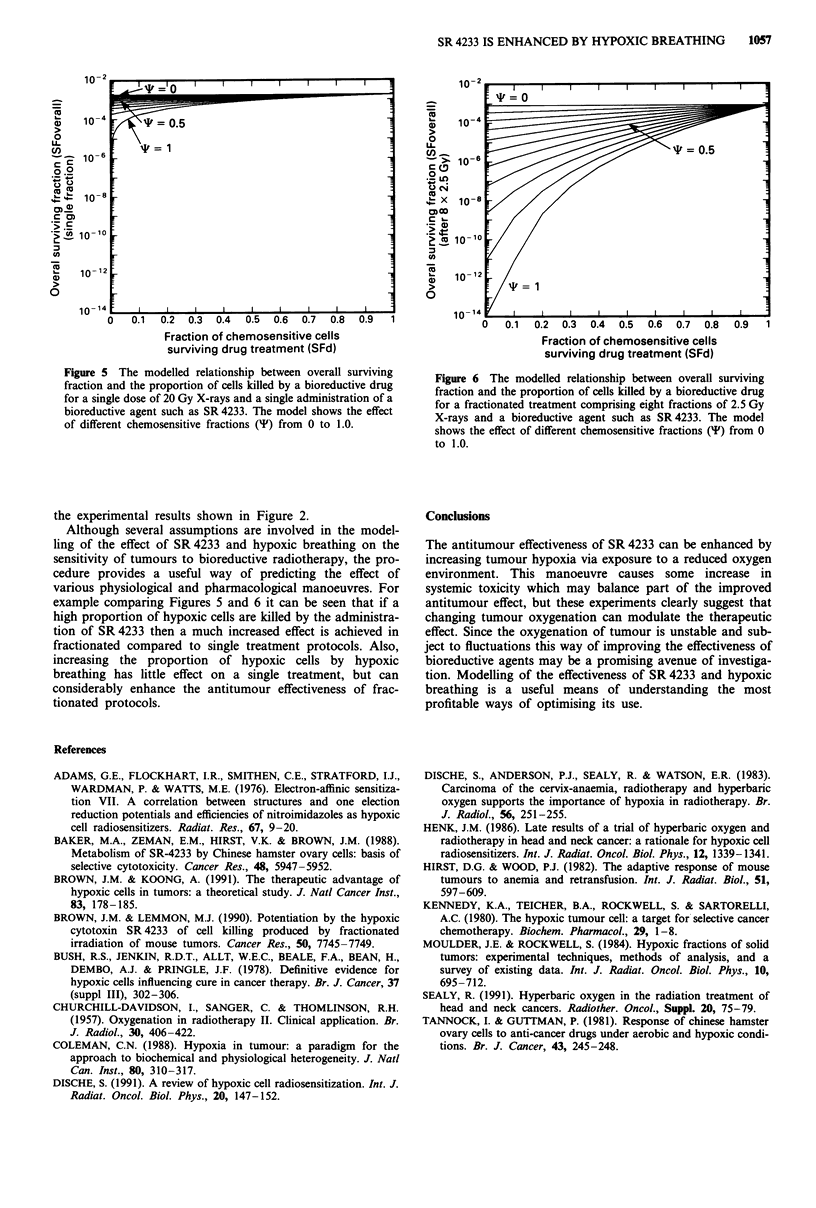
The bioreductive cytotoxic agent SR 4233 (1,2,4-benzotriazine 3-amine 1,4-dioxide) has been shown to markedly potentiate the cell killing of mouse tumours when combined with fractionated radiation therapy. Differential metabolism under oxic compared to hypoxic conditions results in SR 4233 exhibiting selective cytotoxicity to hypoxic cells. This is thought to result from the production of a cytotoxic free radical which is generated predominantly in the absence of oxygen. We have examined a way of enhancing the effectiveness of this antitumour agent in vivo by artificially increasing the hypoxic fraction of tumours by hypoxic breathing. Mice are placed in a chamber containing 10% Oxygen 90% Nitrogen for 1 h after each administration of SR 4233. Our results in the SCCVII tumour model indicate that this manoeuvre results in a 10-fold increase in antitumour effectiveness of SR 4233 when administered in a fractionated regime with radiotherapy (8 x 2.5 Gy and 0.08 mmol kg-1), but not when a single treatment regime (1 x 20 Gy and 0.3 mmol kg-1) is used. Mathematical modelling of this effect is used to illustrate this phenomenon and can be used to predict the dependence of this type of therapy on the modification of tumour oxygenation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. E., Flockhart I. R., Smithen C. E., Stratford I. J., Wardman P., Watts M. E. Electron-affinic sensitization. VII. A correlation between structures, one-electron reduction potentials, and efficiencies of nitroimidazoles as hypoxic cell radiosensitizers. Radiat Res. 1976 Jul;67(1):9–20. [PubMed] [Google Scholar]
- Baker M. A., Zeman E. M., Hirst V. K., Brown J. M. Metabolism of SR 4233 by Chinese hamster ovary cells: basis of selective hypoxic cytotoxicity. Cancer Res. 1988 Nov 1;48(21):5947–5952. [PubMed] [Google Scholar]
- Brown J. M., Koong A. Therapeutic advantage of hypoxic cells in tumors: a theoretical study. J Natl Cancer Inst. 1991 Feb 6;83(3):178–185. doi: 10.1093/jnci/83.3.178. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Lemmon M. J. Potentiation by the hypoxic cytotoxin SR 4233 of cell killing produced by fractionated irradiation of mouse tumors. Cancer Res. 1990 Dec 15;50(24):7745–7749. [PubMed] [Google Scholar]
- Bush R. S., Jenkin R. D., Allt W. E., Beale F. A., Bean H., Dembo A. J., Pringle J. F. Definitive evidence for hypoxic cells influencing cure in cancer therapy. Br J Cancer Suppl. 1978 Jun;3:302–306. [PMC free article] [PubMed] [Google Scholar]
- CHURCHILL-DAVIDSON I., SANGER C., THOMLINSON R. H. Oxygenation in radiotherapy. II. Clinical application. Br J Radiol. 1957 Aug;30(356):406–422. doi: 10.1259/0007-1285-30-356-406. [DOI] [PubMed] [Google Scholar]
- Coleman C. N. Hypoxia in tumors: a paradigm for the approach to biochemical and physiologic heterogeneity. J Natl Cancer Inst. 1988 May 4;80(5):310–317. doi: 10.1093/jnci/80.5.310. [DOI] [PubMed] [Google Scholar]
- Dische S. A review of hypoxic cell radiosensitization. Int J Radiat Oncol Biol Phys. 1991 Jan;20(1):147–152. doi: 10.1016/0360-3016(91)90151-s. [DOI] [PubMed] [Google Scholar]
- Dische S., Anderson P. J., Sealy R., Watson E. R. Carcinoma of the cervix--anaemia, radiotherapy and hyperbaric oxygen. Br J Radiol. 1983 Apr;56(664):251–255. doi: 10.1259/0007-1285-56-664-251. [DOI] [PubMed] [Google Scholar]
- Henk J. M. Late results of a trial of hyperbaric oxygen and radiotherapy in head and neck cancer: a rationale for hypoxic cell sensitizers? Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1339–1341. doi: 10.1016/0360-3016(86)90167-7. [DOI] [PubMed] [Google Scholar]
- Hirst D. G., Wood P. J. The adaptive response of mouse tumours to anaemia and retransfusion. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Apr;51(4):597–609. doi: 10.1080/09553008414552131. [DOI] [PubMed] [Google Scholar]
- Kennedy K. A., Teicher B. A., Rockwell S., Sartorelli A. C. The hypoxic tumor cell: a target for selective cancer chemotherapy. Biochem Pharmacol. 1980 Jan 1;29(1):1–8. doi: 10.1016/0006-2952(80)90235-x. [DOI] [PubMed] [Google Scholar]
- Moulder J. E., Rockwell S. Hypoxic fractions of solid tumors: experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys. 1984 May;10(5):695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- Sealy R. Hyperbaric oxygen in the radiation treatment of head and neck cancers. Radiother Oncol. 1991;20 (Suppl 1):75–79. doi: 10.1016/0167-8140(91)90192-j. [DOI] [PubMed] [Google Scholar]
- Tannock I., Guttman P. Response of Chinese hamster ovary cells to anticancer drugs under aerobic and hypoxic conditions. Br J Cancer. 1981 Feb;43(2):245–248. doi: 10.1038/bjc.1981.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher B. A., Holden S. A., al-Achi A., Herman T. S. Classification of antineoplastic treatments by their differential toxicity toward putative oxygenated and hypoxic tumor subpopulations in vivo in the FSaIIC murine fibrosarcoma. Cancer Res. 1990 Jun 1;50(11):3339–3344. [PubMed] [Google Scholar]
- Van Putten L. M., Kallman R. F. Oxygenation status of a transplantable tumor during fractionated radiation therapy. J Natl Cancer Inst. 1968 Mar;40(3):441–451. [PubMed] [Google Scholar]
- Withers H. R. Biological basis for high-LET radiotherapy. Radiology. 1973 Jul;108(1):131–137. doi: 10.1148/108.1.131. [DOI] [PubMed] [Google Scholar]
- Zeman E. M., Brown J. M., Lemmon M. J., Hirst V. K., Lee W. W. SR-4233: a new bioreductive agent with high selective toxicity for hypoxic mammalian cells. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1239–1242. doi: 10.1016/0360-3016(86)90267-1. [DOI] [PubMed] [Google Scholar]


