Abstract
The fronto-parietal network has been implicated in the processing of multisensory information for motor control. Recent methodological advances wih both fMRI and TMS provide the opportunity to dissect the functionality of this extensive network in humans and may identify distinct contributions of local neural populations within this circuit that are not only related to motor planning, but to goal oriented behaviour as a whole. Herein, we review and make parallels between experiments in monkeys and humans on a broad array of motor as well as non-motor tasks in order to characterize the specific contribution of a region in the parietal lobe, the anterior intraparietal sulcus (aIPS). The intent of this article is to review: 1) the historical perspectives on the parietal lobe, particularly the aIPS; 2) extend and update these perspectives based on recent empirical data; and 3) discuss the potential implications of the revised functionality of the aIPS in relationship to complex goal oriented behavior and social interaction. Our contention is that aIPS is a critical node within a network involved in the higher-order dynamic control of action, including represention of intended action goals. These findings may be important not only for guiding the design of future experiments investigating related issues but may also have valuable utility in other fields, such social neuroscience and biomedical engineering.
Keywords: Goal representation, on-line control, TMS, fMRI, error correction
The parietal cortex has long been thought of as a bridge between perception and action. Mountcastle and colleagues were prescient in noting that neurons in areas 5 and 7 of the non-human primate were not simply sensory in nature, but were involved in higher order sensorimotor integration during hand manipulation tasks within the immediate extrapersonal space for retrieving objects such as food or pulling a lever to receive a juice reward (Mountcastle et al., 1975). In this review we present evidence that the anterior intraparietal sulcus of humans is a key node for hand-object interactions analogous to what is found in the non-human primate. In addition, the data from recent human studies suggest that this region of parietal cortex is not exclusively dedicated to hand-object control. Rather, the site may be critical for representing a diverse range of goal oriented actions.
Hand-Object Interactions
The brain is remarkably adept at orienting the wrist and shaping the span of the fingers to match an object. A particular brain region in the non-human primate parietal cortex is strongly associated with this ability. This region, termed AIP, is found in the anterior-lateral intraparietal sulcus (area 7b), and includes area PFG (von Economo and Koskinas, 1925). Early single unit recordings of macaque AIP indicated a processing role for grasp planning as the firing rate of a substantial number of cells showed a tuning to the type of grip (pincer, power, cone-shaped, etc.) assumed by the animal. The neuronal firing rate of neurons in this area was not simply linked to any particular object. Instead, responses corresponded to the final hand configuration used to grasp the object. An essential involvement in encoding hand configurations was further confirmed by the finding that pharmacological lesions of AIP led to impaired preshaping of the hand toward graspable objects (Gallese et al., 1994). Thus, AIP has come to be viewed as a prototypic region subserving various forms of grasp formation (Murata et al., 2000; Sakata et al., 1992; Sakata et al., 1995; Taira et al., 1990).
Early efforts to identify a human homologue of monkey AIP were hampered by the fact that the human parietal cortex is greatly expanded relative to the primate, and the relationship between Brodmann’s cytoarchitectonic areas (BA 5 and 7) and the intraparietal sulcus (IPS) differed. For example, in the monkey, area 5 comprises part of the superior parietal lobule while area 7 is part of the inferior parietal lobule. In humans, both areas make up the superior parietal lobule. Nevertheless, recent anatomic evidence suggests that neural topography within the IPS of humans and macaque monkeys shows a considerable degree of homology, and thus cross species investigations of the functionality of regions within this sulcus may be a robust way of understanding its role in behavior (Rizzolatti and Matelli, 2003). Initial attempts to localize a human homologue of area AIP within the intraparietal sulcus involved positron emission tomography imaging of cerebral blood flow and during tasks that required grasping objects compared to pointing at objects. Grasping generally induced a relative increase in blood flow in a broad region that encompassed the post-central sulcus. However, the resolution was insufficient to identify a distinct locus of activity within the IPS (Grafton et al., 1996b). With the advent of higher resolution functional magnetic resonance imaging (fMRI) methods and similar tasks, it became possible to localize, in normal subjects making simple prehensile actions, the activation to an anterior portion of the intraparietal sulcus (Binkofski et al., 1998; Culham et al., 2003; Johnson-Frey et al., 2005). Analysis of fMRI results on an individual subject basis further showed that the activation was most consistently located at the junction of the postcentral and intraparietal sulci (Johnson-Frey et al., 2005). Cerebral vascular accidents localized to this region in humans likewise lead to impaired hand preshaping when reaching to grasp objects (Binkofski et al., 1998). More recent studies incorporating complex object manipulation show this region can also be linked to the control of precision grip forces used for grasping everyday small objects (Ehrsson et al., 2001). More generally, anterior intraparietal sulcus (aIPS) in humans has been associated with a broad range of tasks involving cross modal integration, particularly in tasks relating vision and haptics (Grefkes et al., 2002). Table 1 provides a summary of 22 functional imaging studies that involve grasping or cross-modal integration including observation as a task. Figure 1 demonstrates the remarkable and consistent overlap within aIPS for all of these studies.
Table 1.
Published Talairach coordinates of the peak voxel activation in or near the human aIPS as reported by independent investigators using a variety of tasks. Asterisks denote that coordinates have been converted from Montreal Neurological Institute (MNI) (http://www.mrccbu.cam.ac.uk/Imaging/Common/mnispace.shtml). The row numbers (1-22) correspond to the numbers in the color-coded legend in Figure 1, in which these coordinates are superimposed on a high resolution template of a canonical brain.
| Left hemisphere | Right hemisphere | ||||||
|---|---|---|---|---|---|---|---|
| Contrast | Reference | x | y | z | x | y | Z |
| 1. Grasping vs Pointing | (Binkofski et al., 1998) | -45 | -35 | 43 | |||
| 2. Grasping vs Reaching | (Culham et al., 2003) | -38 | -48 | 52 | 40 | -50 | 50 |
| 3. Grasping vs Pointing | (Johnson-Frey et al., 2005) | -40 | -33 | 43 | |||
| 4. Grip force vs No grip force | (Ehrsson et al., 2001) | -40 | -40 | 36 | 48 | -52 | 52 |
| 5. Small grip force vs Large | Ibid. | 52 | -44 | 48 | |||
| 6. Manipulation complex vs simple object | (Binkofski et al., 1999a) | -48 | -34 | 40 | 48 | -34 | 40 |
| 7. Manipulation complex vs simple object | (Binkofski et al., 1999c) | -40 | -40 | 40 | 40 | -40 | 44 |
| 8. Haptic exploration vs Squeezing | (Jancke et al., 2001) | -44 | -40 | 40 | 36 | -44 | 44 |
| 9. Modelling vs Squeezing | Ibid. | -40 | -44 | 40 | 32 | -40 | 40 |
| 10. Haptic exploration vs Imagining | Ibid. | -44 | -32 | 40 | 40 | -32 | 40 |
| 11. Modelling vs Imagining | Ibid. | -48 | -32 | 40 | 40 | -40 | 40 |
| 12. Crossmodal vs Unimodal matching | Grefkes et al, 2002 | -42 | -38 | 38 | |||
| 13. Viewing tools vs Other categories | (Chao and Martin, 2000) | -32 | -47 | 42 | |||
| 14. Naming tools vs Other categories | Ibid. | -30 | -39 | 47 | |||
| 15. Orientation vs Color discrimination | (Shikata et al., 2001) | -37 | -40 | 47 | 45 | -30 | 52 |
| 16. Orientation vs Color discrimination | (Shikata et al., 2003) | -36 | -39 | 39 | 39 | -39 | 39 |
| 17. Imagine grasp vs Orientation discrimination | Ibid. | -39 | -39 | 51 | 45 | -39 | 39 |
| 18. Pantomined grasp vs Imagined grasp | Ibid. | -30 | -45 | 45 | 45 | -30 | 39 |
| 19. Action observation vs Name object | (Shmuelof and Zohary, 2005) | -37 | -44 | 50 | 35 | -44 | 50 |
| 20. R-hand, L-object vs L-hand, R-object | Ibid. | -36 | -42 | 54 | |||
| 21. L-hand, R-object vs R-hand, L-object | Ibid. | 32 | -37 | 55 | |||
| 22. Repetition supression for goal | (Hamilton and Grafton, 2006)* | -47 | -34 | 37 | |||
| Mean | -39.7 | -39.3 | 43.2 | 41.1 | -39.7 | 44.8 | |
| Standard deviation | 5.4 | 4.8 | 5.3 | 6.0 | 6.6 | 5.8 | |
Figure 1.
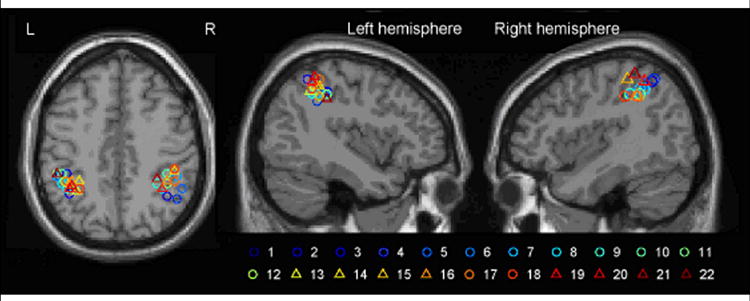
A meta-analysis of activations reported in the published literature for a variety of perceptual and motor tasks involving reach-to-grasp movements. Each circle (and the respective color-coded number) represents the locus of the peak activation in the corresponding grasp study and task listed in Table 1. Triangles correspond to grasp observation and related studies. The locations are superimposed on a high resolution template of the single_subj_T1 brain provided in the SPM package. The sections are shown at: axial, z = 44; left sagittal, x = -40; right sagittal, x = 41; Talairach coordinates.
These aggregate studies make two very important advances. First, they establish a reliable bridge between non-human and human functional neuroanatomy (Grefkes and Fink, 2005). This provides a stepping stone for future studies using monkey and human brains as complementary systems. Second, these studies provide compelling evidence that in humans, like in monkeys, aIPS is critical for planning and/or controlling a broad range of grasp related actions. What is uncertain from these initial human imaging studies is whether the aIPS functions solely as a look-up table for determining grasp configurations based on perceptual features, or if this region has a broader role in the dynamic control of actions and goals. In the following sections we argue that aIPS is central to the higher-order control of actions.
Dynamic control of action
In addition to a purported role in planning, AIP in the macaque is likely to be essential for the on-line control of grasping. Upon object contact, the fingers rapidly adapt to the objects surface in order to generate smooth and coordinated grip force (Brochier et al., 2004; Smith et al., 1993). This on-line control requires the rapid integration of the motor command, the current 3-dimensional estimate of an object’s shape, mass and other properties, and afferent information (Gardner et al., 2006). The rich connections that AIP shares with other parietal regions, as well as with the occipital and frontal cortices places it in a strategic position for multimodal integration. It is entirely plausible that macaque AIP is the key locus for integrating these different sources of information to form continuous estimates of the state of the system.
A key question is whether human aIPS is more than a sensory integration area. Is it also essential for the dynamic on-line control of grasping and related actions? This proposition gains support from evidence that lesions of human posterior parietal cortex lead to impaired on-line control of reaching (Grea et al., 2002; Pisella et al., 2000). In a dramatic example, one subject, when faced with a perturbation of target location during an ongoing movement, completed an already planned grasping movement at the original location, then plan a second movement to the new location. In addition, transcranial magnetic stimulation (TMS) disruption in the region of aIPS leads to impaired on-line control for reaching in a target perturbation task (Desmurget et al., 1999). Recently, our laboratory conducted a series of experiments to specifically identify the role of aIPS in two interacting processes: the dynamic control of grasp and the representation of a grasp related goal. We used TMS to generate virtual lesions in healthy human subjects to investigate dynamic control because TMS offers temporal precision not possible with fMRI and, unlike fMRI, it can attribute causality between brain anatomy and function. In all tasks aIPS was defined anatomically on each subject’s high resolution structural MRI at the junction between the anterior extent of the IPS and the inferior postcentral sulcus (Figure 2). TMS pulses were delivered to aIPS, as well as other cortical control sites, as subjects reached-to-grasp a rectangular object. A fast motor was used to rotate the target object, on a trial-by-trial basis, by either 180° (on 75% of the trials) or by 90° on randomly selected trials, from an initial horizontal orientation. Because participants were instructed to always grasp the object along an imaginary vertical dimension, the grasp aperture requirement remained unperturbed in the 180° trials but increased from 2 to 10 cm in the 90° trials (Figure 3, left insets). In a second experiment, participants were asked to always grasp the object along its narrow dimension, irrespective of the object’s orientation (Figure 3, right insets). Note that the two tasks differed only in the motor requirements needed to mediate the adaptive response, i.e. finger flexors-extensors to adapt aperture in the first task and forearm pronators-supinators to adapt forearm orientation in the second task. All other factors, including the object orientation remained identical.
Figure 2.

Location of TMS stimulation to aIPS in Tunik et al. (2005) and Rice et al. (Rice et al., 2006).
Figure 3.

Effect of TMS on on-line control of grasp as a function of aperture size (left) and hand orientation (right)). Results adapted from Tunik et al. (2005).
Three noteworthy findings emerged. First, TMS to the aIPS site, and not to any other cortical sites, produced a delay in the adaptive response of the perturbed relative to unperturbed trials (Figure 3, left, blue solid line). This effect was contingent on the timing of the TMS pulse being locked to the occurrence of the perturbation, and was not evident when TMS was delivered at large delays after the perturbation, near the time of object contact. Second, the TMS-induced delay in adaptation was present for adapting the grasp aperture as well as for adapting the forearm orientation – adaptive responses which are mediated by completely different effectors. Third, only the time required to actually grasp the object was affected by the TMS, not the time required to reach the target (Tunik et al., 2005). The data from these experiments challenges the view that aIPS is simply a repository for grasp configurations and instead makes a convincing case that aIPS is a flexible dynamic site capable of representing action goals independent of effectors, and one that is highly involved in online control. Our contention from this study is that aIPS may perform iterative comparisons during an ongoing movement between an efference copy of the motor command and incoming sensory information in order to assure that the current grasp plan matches the current context and sensorimotor state (Tunik et al., 2005). However, because visual feedback was continuously available, a limitation of these experiments was our inability to dissociate where on a perceptual – motor landscape the computations performed by aIPS may be positioned.
In a series of follow-up experiments, we addressed this in order to understand the specific computations that may be performed by aIPS (Rice et al., 2006). Participants performed a similar reach-to-grasp task and liquid crystal spectacles (Translucent Technologies, Inc.) were used to limit the initial viewing period to 200ms. During this period, the object was visible either in a horizontal or in a vertical orientation. Thus, subjects were required to make a reach-to-grasp plan on a trial-to-trial basis. Movement initiation was signalled by the release of a start button held between trials. At button release one of two things happened. In experiment 1, the object remained unperturbed and the glasses remained opaque (see Figure 4a). In this case, subjects simply executed the plan that they made during the viewing period. Double-pulsed TMS (100 ms inter-stimulus interval) was delivered either during viewing period (movement planning) or synchronously with the release of the button (movement execution). This experiment addressed the issue of whether aIPS computes adaptive responses for perturbations per se, or whether aIPS is more broadly involved in continuous monitoring of movements in general, irrespective of an explicit need to update. In experiment 2, just after the initial viewing period, the object’s aperture was perturbed (either from large to small or small to large) and a second viewing period (200ms) was provided synchronously with movement initiation to inform subjects of the perturbation (see Figure 4d). In this experiment, double pulse TMS was delivered either during this feedback epoch (error detection) or immediately after (error correction). In other words, control of the viewing allowed us to dissociate perceptual from corrective updating processes. Time-locking the TMS pulses to the viewing and post-viewing epochs in experiment 2 allowed us to specifically dissociate perceptual from executive, respectively, control processes. In these studies two novel findings emerged. First, TMS-induced deficits were evident in the no-perturbation task as well as the perturbation task, suggesting the computations in aIPS are not limited to an explicit need to update the movement. Second, the deficits were produced only in the movement execution phase in the first experiment when there was no perturbation (Figure 4, b and c) and only during the error correction phase in the second experiment (Figure 4, e and f). TMS had no effect when it was applied prior to movement onset, suggesting that aIPS is not merely involved in the initial perceptual evaluation of an object features. Interestingly, in a subset of subjects tested on the original perturbation task, the corrective computations performed within aIPS occured very rapidly, i.e. within 65 ms after the completion of the perturbation (Tunik et al., 2005).
Figure 4.

Effect of TMS on grasp control during planning and execution of unperturbed (A-C) and perturbed (D-F) trial types. Results adapted from Rice et al. (2006)
A traditionally held view from neurophysiological recordings is that aIPS is a repository of grip apertures generated from object features. Two dimensional features of images projected onto the retina such as object shape, size, and orientation have been found to be encoded not only in early visual areas, but also by neurons in monkey area AIP (Murata et al., 2000). These 2D features are used by the CNS to construct 3D representations of objects. As such, neurons representing 3D shape have been found in the caudal intraparietal sulcus (area CIP) (Sakata et al., 2005; Tsutsui et al., 2005) as well as in the anterior intraparietal sulcus (area AIP) (Sakata et al., 2005) of monkeys. Paralleling the monkey data are human neuroimaging studies showing intraparietal sulcus activation in response to perceptual tasks involving manipulation of object shape and orientation (Culham and Valyear, 2006). Given this sensitivity to object features, it might be concluded that human aIPS should be considered as a purely perceptual area. Our TMS studies suggest this is an oversimplification. Instead we propose that aIPS makes a specific contribution to grasping control by performing an on-line computation of a difference vector based on motor goal, efference copy and sensory inputs. It would be difficult to imagine how aIPS could possibly be involved in this dynamic control of context-specific action if it was not sensitive to the 3D world that the actor operates in. However, dynamic control requires more than just the recognition of object features. The generalization of aIPS function to dynamic control is also supported by the proposal that a portion of the human aIPS is evolutionarily newer than its putative monkey homolog area AIP (Orban et al., 2005). Orban and colleagues suggest that this may underlie its more complex role in hand-object and hand-tool interactions. Our data are consistent with this hypothesis.
We propose that computation in aIPS is not a low-level red flag that simply signals a mismatch between these sources of information. It is more likely that aIPS is outputting either an evaluative description of the mismatch, i.e. a difference vector, or perhaps even a solution to resolve it. Although interference within this site produces deficits in grip aperture when the goal is to control aperture, it also induces deficits in forearm orientation when this is the goal at hand. Furthermore, TMS to adjacent sites along the sulcus likewise disrupts online adaptation during grasping (Glover et al., 2005) as well as reaching in the presence of visual (Desmurget et al., 1999) and force (Della-Maggiore et al., 2004) perturbations. A parsimonious explanation is that aIPS, and adjacent regions within the IPS, may be a repository for a broad range of motor representations that lead to successful hand-object interactions based on shape, orientation, weight, surface texture, location and so on. Our experiments clearly show that aIPS is performing dynamic, goal-based, sensorimotor transformations that involve at least 3 variables: the current sensory state (context), the current motor command, and the current goal. This function is highly reliant on the integrity of the parietal lobe and is an example of the establishment of an internal representation of an action, also referred to as an internal model by some investigators (Desmurget and Grafton, 2000; Sirigu et al., 1996; Wolpert et al., 1998). The notion of an internal model is based on computational principles and there are multiple ways in which delayed feedback and feedforward commands might be integrated in the parietal cortex to enhance control (Wolpert and Ghahramani, 2000). At this point, our data cannot speak to which if any of these models put forth in the literature is instantiated in the cortex.
Beyond on-line control of grasp
Results from recent unit recordings in monkey area PFG, a region just inferior to AIP on the lateral convexity of the inferior parietal lobule, suggest that single neurons in this region are selective for not just the current grasping action, but also the subsequent movements to be performed, which could be considered as the overall goal (Fogassi et al., 2005). These results raise the possibility that human aIPS and adjacent cortex may likewise represent actions at a hierarchically higher level, representing goals rather than grasps (Fogassi et al., 2005). Functional neuroimaging data has shown that aIPS has a role beyond grasping (Culham et al., 2006; Grefkes and Fink, 2005). Binkofski (1999a;1999b) demonstrated that the manipulation of complex meaningless objects, versus simple objects leads to activation in a network of regions including aIPS. aIPS is active during tactile exploration of objects as well as modelling an object (i.e. constructing objects) (Jancke et al., 2001). A role of aIPS in crossmodal processing has also been identified by Grefkes, (2002), who showed increased neural activity in aIPS when subjects transferred information between visual and tactile modalities. A region in the vicinity of aIPS shows activation during viewing and naming of tools, when compared to other stimuli such as animals, faces and houses (Chao and Martin, 2000). Finally, this region, along with adjacent IPL, is recruited on an array of grasp observation tasks (Grafton et al., 1996a), object orientation discrimination tasks (Shikata et al., 2001; Shikata et al., 2003), and grasp imagery and pantomime tasks (Shikata et al., 2003) (See Table 1 and Fig. 1 for Talairach co-ordinates for activation loci in the cited literature).
Hemispheric Specialization?
A number of studies have used TMS and neuroimaging to address the issue of hemispheric specialization for perception and action in the anterior portion of the intraparietal sulcus. A first reasonable question is whether left-right aIPS specialization might be parcelled according to action representations in intrinsic (joint space) versus extrinsic (location of the object in external space) coordinate frames. Some evidence suggests that this may be the case. Using an action observation task, Shmuelof and Zohary (2006) found that BOLD activity in left aIPS was predominantly driven by observing right (contralateral) hand-object interactions, irrespective of visual field (left or right) in which the interaction occurred. Conversely, they found that right aIPS was driven by both, the contralateral observed hand as well as the visual field in which the hand-object interaction occurred. Thus, that study reveals a left aIPS specialization for representing the contralateral acting hand. The right aIPS likewise represented the contralateral hand, though this representation was also sensitive to the visual field in which the interaction occurred. Rushworth et al., (2001) have posited that the anterior portion of IPS in the left hemisphere may be specialized for what they called “motor attention”, a term that bears similarity with the data of Schmeulof and Zohary (2006)as well as our depiction of aIPS function.
While the above results suggest a degree of left aIPS specialization, the issue remains unresolved. For example, several neuroimaging studies have systematically shown left hemisphere aIPS activation using action understanding paradigms (Hamilton and Grafton, 2006; Hamilton et al., 2006; Iacoboni et al., 2005). When reviewing motor paradigms, the matter of functional lateralization in aIPS remains murky. Most studies show bilateral activation in aIPS during grasping, with a stronger activation in the aIPS contralateral to the grasping hand. (Binkofski et al., 1998; Culham et al., 2003) although one showed only contralateral recruitment (Johnson-Frey et al., 2005). Finally, studies which have applied TMS in the vicinity of aIPS during pointing movements with either arm have yielded inconsistent results. Desmurget et al (1999) only observed deficits in right-arm pointing in response to left IPS stimulation while (Vesia et al., 2006) elicited left IPS-induced deficits when pointing was performed by either arm. The above evidence and our own experience allows us to unequivocally attribute right hand-object interactions to the left aIPS. However, more systematic investigations into the laterality issue are needed before any definitive conclusion can be made as to the specializations within the left and right aIPS.
Action goals
Intention or motor goal related activity at the level of single neurons has been demonstrated in multiple areas of monkey posterior parietal cortex including the “parietal reach region” (Andersen and Buneo, 2002; Batista and Andersen, 2001). An obvious question is whether similar goal related activity can be observed in the IPS of humans and to determine the specificity of this goal representation. This is a challenging problem to address with functional imaging because goals and intentions are embedded in all actions. It is difficult to create plausible motor paradigms that specifically manipulate the presence or absence of a goal or intention. Such manipulations are needed with conventional subtraction methodology in fMRI. A recent approach to neuroimaging, known as repetition suppression (RS) or functional magnetic resonance-adaptation (fMR-A) provides an elegant alternative strategy for addressing this challenge. RS is based on the finding that repeated presentation of a particular stimulus feature will lead to a reduction in the BOLD signal in the region where that feature is encoded (Grill-Spector and Malach, 2001). This approach has been used in two recent studies investigating action recognition and observation. Shmuelof (2005) showed participants blocks of pictures with repeated objects or repeated grasps, or both. They reported suppression specific to both the type of grasp and the object to be grasped in the left aIPS, which suggests that this region is not sensitive only to grasp configuration. In a recent study from our laboratory (Hamilton and Grafton, 2006), we used a repetition suppression design to determine the neural correlates of goal representation. In this study participants watched a sequence of video clips of a hand reaching and grasping one of two objects placed in one of two locations. Each video clip in the sequence was defined in relation to the previous clip (a one-back repetition suppression design) as one of four possible conditions (1) Repeated goal, repeated trajectory; (2) Repeated goal, novel trajectory; (3) Novel goal, novel trajectory; (4) Novel goal, novel trajectory (see Figure 5). We predicted that brain regions which encode the goal of the observed action should show reduced responses to repeated goals compared to novel goals, regardless of the hand trajectory. This pattern of response was found in two regions within the intraparietal sulcus, including aIPS (i.e. neuronal response decreased when second video clip was presented with the same goal, regardless of trajectory). This provides evidence that aIPS is sensitive to goals of actions.
Figure 5.
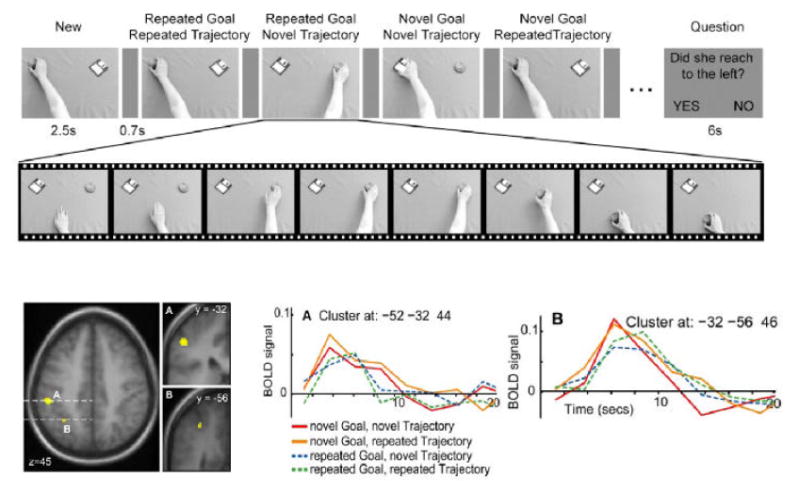
Repetition suppression for goal in human aIPS. A. The stimulus sequence used to induce suppression in a one-back design. Each image indicates a movie of a hand reaching out and taking an object. B. Clusters in parietal cortex which showed specific suppression when the goal of the action was repeated. The larger cluster in aIPS survived the p<0.05 corrected threshold. C. The post-stimulus response in the aIPS cluster for each condition. Stronger responses are seen to novel goals (red and orange) compared to repeated goals (blue and green), regardless of hand trajectory.
The studies conducted so far on goal representations in aIPS have used very simple, object goals, where the intention of the actor is to take a particular object. In daily life, we accomplish a variety of long and short term goals which often involve multiple interactions with multiple objects. It is not yet known if the parietal cortex also has a role in sequencing multiple simple goals to accomplish more complex behaviours, or if this is a function of other regions such as the frontal cortex (Shallice and Burgess, 1991). Work to address this question is ongoing in our lab.
Goals for self and others
The data presented above demonstrate that aIPS encodes the goal of observed actions (Hamilton and Grafton, 2006) and performed actions (Tunik et al., 2005). Studies of action execution, observation and imitation demonstrate recruitment of a common set of inferior frontal, premotor and parietal cortical areas that have been designated the mirror neuron system (Rizzolatti and Craighero, 2004). Thus, it seems that the principle of mirroring, that is, common representations for features of the self and of other people – applies in aIPS. This is plausible as aIPS is adjacent to the inferior parietal region where mirror neurons for action sequences have been recorded in the macaque (Fogassi et al., 2005). We now propose that in humans, aIPS provides a higher-order level goal representation, for both performed and observed actions. This hypothesis has implications for our interpretation of the mirror system and its mode of operation.
If goals are represented in aIPS, what are we to make of the alternative claims motivated by studies of human mirror neuron circuits that the inferior frontal gyrus is the principal area to encode goals? The two regions are densely interconnected, but may not have identical functions. Two studies imply a role for IFG rather than aIPS in goal representation. Single neurons within the IFG respond when a monkey can infer that a human is grasping an object behind a screen (Umilta et al., 2001). However, this experiment did not distinguish between goals and kinematic parameters, and thus the neurons could be responding to the inferred grasp characteristics, rather than the goal of the action. Meanwhile, evidence linking human IFG to intentions is derived from an fMRI study where actions performed in a context resulted in greater IFG activity than isolated actions (Iacoboni et al., 2005). However, isolated actions do not lack an intention, so it is not clear what this subtraction reveals. Furthermore, it is hard in this case to exclude the possibility that ‘canonical neurons’ which respond to objects alone, are the driving force behind the context effects. Finally, it is not clear why authors find stronger activation for drinking actions compared to cleaning actions, when both are likely to be equally common in daily life. To summarise, we do not contest the findings that IFG is intimately connected with aIPS and tends to be coactivated with the parietal cortex in a range of action observation studies (i.e. Aziz-Zadeh et al., 2006). However, we suggest that there is strong evidence for aIPS as a centre for intermediate, or object centered goals, and that IFG may have an alternative function that is beyond the scope of this review.
Second, the presence of a representation of observed goals in aIPS raises the question of how these goals might be computed from the visual array. One possibility is that goals are inferred by ‘direct matching’ or ‘resonance’ where visual information is mapped to low level motor representations, and goals are then inferred from the motor stage (Gallese et al., 2004). If true, then one might expect to see a dependency on the body part used to perform an action (Buccino et al.). An alternative is visual inputs to aIPS from regions such as the superior temporal sulcus provide an abstract visual representation of the action, from which the goal can be extracted by emulation (Csibra, 2006). This possibility is supported by the finding that parietal regions respond to the observation of biting actions performed by dogs or monkeys (Buccino et al., 2004), even though the low level motor parameters must differ greatly between humans and dogs. However, this topic remains controversial, and further research will be needed to determine how goal-level representations of observed actions are formed in aIPS, how this brain region responds to goals outside the observers repertoire and to distinguish the roles of direct matching and emulation models of action understanding.
Implications
The data from our and other independent laboratories strongly indicate that the rostral extent of the inferior parietal lobule encompassing the anterior portion of the intraparietal sulcus, is critically involved in a broad range of functions that extend beyond the basic control of preshaping the hand to match a target object. Specifically, empirical data indicate that the dynamic role of aIPS in online control is: 1) context (goal)-dependent rather than effector-dependent; 2) critical during the dynamic evolution of the movement rather than during earlier perceptual phases; and 3) shows repetition suppression effects to action goals. We interpret this as evidence that aIPS (and possibly the PFG homologue) may be situated near the top of a motor action hierarchy. This puts this region in three major positions: 1) to set action goals; 2) to perceive action goals/intentions; and 3) to modulate or entrain downstream action circuits. The implications of these possible functions are discussed below.
Potential role in skill learning
One noteworthy finding is the apparent sensitivity of the aIPS to repetition suppression effects when an individual repeatedly observes similar objects and hand configurations (Shmuelof and Zohary, 2005) as well as action goals (Hamilton and Grafton, 2006). An implication of this is that aIPS may function as a short-term context-specific information capacitor for action. We have previously argued that aIPS may perform online integration of an intended action goal, the efferent motor command, and incoming (re)afferent input regarding the ongoing sensorimotor context (Tunik et al., 2005). Perhaps the integration of these inputs within aIPS generates a difference vector that is maintained as a training signal from one event to another. Conceptually, naiveté on a given task should be associated with a large difference vector that would become reduced with experience. Motor experience-based reduction of activity has been identified within this region (Handy et al., 2006) when subjects observe graspable objects, and this reduction may be due to a long term repetition suppression effect. If true, then in addition to the role that aIPS plays for dynamic, online, control of action, it may equally be important for trial-to-trial adaptation. While this thesis has not yet been directly tested, indirect evidence suggests that a region adjacent to aIPS may be involved in just such trial-to-trial adaptation (Della-Maggiore et al., 2004). In this latter study, TMS of parietal cortex was used to disrupt adaptation. However the use of TMS on every trial made it impossible to dissociate impaired online performance from trial-to-trial adaptation. A prediction of the trial-to-trial adaptation thesis is that disrupting activity within aIPS during an action (i.e. by using TMS) should affect performance not only on the concurrent (TMS) trial, but also on the subsequent (non-TMS) trial.
Potential targets for implantation of neural prosthetic controllers
Direct motor output regions, such as in the primary motor cortex (M1), are conventional targets for implanting microelectrode arrays used in controlling neural prostheses (Schwartz, 2004) because of the high fidelity between the correlated activity of neural populations in M1 and various movement variables (i.e. velocity, direction, and force) (Reina et al., 2001; Sergio et al., 2005). In the future it may become more important to identify targets for neural prostheses directed towards areas that represent action goals, rather than lower level kinematics. The current review suggests that aIPS may become a plausible location for such a device.
aIPS in social interaction
Recent work on observation of actions in humans and monkeys has lead to the concept of a mirror neuron system in the inferior frontal and inferior parietal cortex, which responds to both the performance and observation of actions (Rizzolatti and Craighero, 2004). These results have also provided the basis for several speculative proposals concerning the role of motor systems in ‘direct-matching’ between self and other (Iacoboni et al., 1999) and in inferences about the goals, intentions, desires and beliefs of other people (Gallese et al., 2004). The finding that aIPS, an area known to be part of the putative human mirror neuron system, represents the goals of other people’s actions (Hamilton and Grafton, 2006) could be taken as evidence in favor of these proposals. In particular, our fMRI and TMS experiments converge on the notion that aIPS encodes the goals of one’s own actions (Tunik et al., 2005) and of other people’s actions (Hamilton and Grafton, 2006), which is coherent with the concept of a common representational system for the actions of self and other. As a centre for interpreting other people’s behaviour, aIPS could thus be considered part of the social brain. However, there are several reasons to be cautious about accepting the mirror neuron hypothesis wholesale. First, the idea of ‘direct-matching’ between self and other seems overly simplistic. Our data indicate that only one small portion of the IPL – IFG circuit demonstrated RS for action goals, suggesting other regions may have different functions. Thus, it may not make sense to speak of a unitary mirror neuron system. Further studies will be needed to understand how different parietal and frontal components contribute to action understanding. In particular, it is important to remember that these regions are fundamentally movement related regions, organised in a sophisticated motor hierarchy and with an essential role in the ongoing control of ones own action. Understanding the actions of other people may be a secondary function, built onto and subservient to, the motor hierarchy. For example, performing an action can systematically bias participants’ perception of another person’s action (Hamilton et al., 2004; Hamilton et al., 2006), indicating that motor processing may take precedence. Second, inferences from actions and goals to mental states such as belief and desire are by no means simple (Jacob and Jeannerod, 2005). Goal representations in aIPS may provide one piece of information contributing to online inferences about beliefs and desires, but there are many other sources of information about other people’s mental states (Frith and Frith, 2006), and further research will be needed to determine how these interact.
Conclusion
Our principle argument is that aIPS, the human homologue of area AIP in the monkey is a region whose function far exceeds a low-level representation of grasp configurations. Instead, empirical findings suggest that aIPS is critically involved in dynamic control of action at a goal level. This hypothesis has important implications for motor skill learning, with applications to the field of neurorehabilitation, as well for theories pertaining to social neuroscience.
Acknowledgments
Supported by PHS grants NS 33504, NS 44393 and the James S. McDonnell Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Koski L, Zaidel E, Mazziotta J, Iacoboni M. Lateralization of the human mirror neuron system. J Neurosci. 2006;26:2964–2970. doi: 10.1523/JNEUROSCI.2921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista AP, Andersen RA. The parietal reach region codes the next planned movement in a sequential reach task. J Neurophysiol. 2001;85:539–544. doi: 10.1152/jn.2001.85.2.539. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund H. A fronto-parietal circuit for object manipulation in man: evidence from an fMRI-study. Eur J Neurosci. 1999a;11:3276–3286. doi: 10.1046/j.1460-9568.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Stephan KM, Rizzolatti G, Seitz RJ, Freund HJ. A parieto-premotor network for object manipulation: evidence from neuroimaging. Exp Brain Res. 1999b;128:210–213. doi: 10.1007/s002210050838. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Classen J, Benecke R. Stimulation of peripheral nerves using a novel magnetic coil. Muscle Nerve. 1999c;22:751–757. doi: 10.1002/(sici)1097-4598(199906)22:6<751::aid-mus12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Dohle C, Posse S, Stephan KM, Hefter H, Seitz RJ, Freund HJ. Human anterior intraparietal area subserves prehension: a combined lesion and functional MRI activation study. Neurology. 1998;50:1253–1259. doi: 10.1212/wnl.50.5.1253. [DOI] [PubMed] [Google Scholar]
- Brochier T, Spinks RL, Umilta MA, Lemon RN. Patterns of muscle activity underlying object-specific grasp by the macaque monkey. J Neurophysiol. 2004;92:1770–1782. doi: 10.1152/jn.00976.2003. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- Buccino G, Lui F, Canessa N, Patteri I, Lagravinese G, Benuzzi F, Porro CA, Rizzolatti G. Neural circuits involved in the recognition of actions performed by nonconspecifics: an FMRI study. J Cogn Neurosci. 2004;16:114–126. doi: 10.1162/089892904322755601. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. Neuroimage. 2000;12:478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Csibra G. Action mirroring and action understanding: An alternative account. In: Rossetti Y, Kawato M, Haggard P, editors. Attention and Performance, XXII. 2006. (In Press) [Google Scholar]
- Culham JC, Danckert SL, DeSouza JF, Gati JS, Menon RS, Goodale MA. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp Brain Res. 2003;153:180–189. doi: 10.1007/s00221-003-1591-5. [DOI] [PubMed] [Google Scholar]
- Culham JC, Cavina-Pratesi CC, Singhal A. The role of parietal cortex in visuomotor control: What have we learned from neuroimaging? Neuropsychologia. 2006;44:2668–2684. doi: 10.1016/j.neuropsychologia.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Culham JC, Valyear KF. Human parietal cortex in action. Curr Opin Neurobiol. 2006;16:205–212. doi: 10.1016/j.conb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Della-Maggiore V, Malfait N, Ostry DJ, Paus T. Stimulation of the posterior parietal cortex interferes with arm trajectory adjustments during the learning of new dynamics. J Neurosci. 2004;24:9971–9976. doi: 10.1523/JNEUROSCI.2833-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat Neurosci. 1999;2:563–567. doi: 10.1038/9219. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci. 2000;4:423–431. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren E, Forssberg H. Differential fronto-parietal activation depending on force used in a precision grip task: an fMRI study. J Neurophysiol. 2001;85:2613–2623. doi: 10.1152/jn.2001.85.6.2613. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. How we predict what other people are going to do. Brain Res. 2006;1079:36–46. doi: 10.1016/j.brainres.2005.12.126. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends Cogn Sci. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Gallese V, Murata A, Kaseda M, Niki N, Sakata H. Deficit of hand preshaping after muscimol injection in monkey parietal cortex. NeuroReport. 1994;5:1525–1529. doi: 10.1097/00001756-199407000-00029. [DOI] [PubMed] [Google Scholar]
- Gardner EP, Babu KS, Reitzen SD, Ghosh S, Brown AM, Chen J, Hall AL, Herzlinger M, Kohlenstein JB, Ro JY. Neurophysiology of prehension: I. Posterior parietal cortex and object-oriented hand behaviors. J Neurophysiol. 2006 doi: 10.1152/jn.00558.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover S, Miall RC, Rushworth MF. Parietal rTMS disrupts the initiation but not the execution of on-line adjustments to a perturbation of object size. J Cogn Neurosci. 2005;17:124–136. doi: 10.1162/0898929052880066. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp representations in humans by positron emission tomography. 2. Observation compared with imagination. Exp Brain Res. 1996a;112:103–111. doi: 10.1007/BF00227183. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fagg AH, Woods RP, Arbib MA. Functional anatomy of pointing and grasping in humans. Cereb Cortex. 1996b;6:226–237. doi: 10.1093/cercor/6.2.226. [DOI] [PubMed] [Google Scholar]
- Grea H, Pisella L, Rossetti Y, Desmurget M, Tilikete C, Grafton S, Prablanc C, Vighetto A. A lesion of the posterior parietal cortex disrupts on-line adjustments during aiming movements. Neuropsychologia. 2002;40:2471–2480. doi: 10.1016/s0028-3932(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. J Anat. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Weiss PH, Zilles K, Fink GR. Crossmodal processing of object features in human anterior intraparietal cortex: an fMRI study implies equivalencies between humans and monkeys. Neuron. 2002;35:173–184. doi: 10.1016/s0896-6273(02)00741-9. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol (Amst) 2001;107:293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Wolpert D, Frith U. Your own action influences how you perceive another person’s action. Curr Biol. 2004;14:493–498. doi: 10.1016/j.cub.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Grafton ST. Goal representation in human anterior intraparietal sulcus. J Neurosci. 2006;26:1133–1137. doi: 10.1523/JNEUROSCI.4551-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AF, Wolpert DM, Frith U, Grafton ST. Where does your own action influence your perception of another person’s action in the brain? Neuroimage. 2006;29:524–535. doi: 10.1016/j.neuroimage.2005.07.037. [DOI] [PubMed] [Google Scholar]
- Handy TC, Schaich-Borg J, Turk DJ, Tipper CM, Grafton ST, Gazzaniga MS. Motor experience with graspable objects reduces their implicit analysis in visual and motor-related cortex. Cogn Brain Res. 2006 doi: 10.1016/j.brainres.2006.04.059. In Press. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one’s own mirror neuron system. PLoS Biol. 2005;3:e79. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Jacob P, Jeannerod M. The motor theory of social cognition: a critique. Trends Cogn Sci. 2005;9:21–25. doi: 10.1016/j.tics.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Jancke L, Kleinschmidt A, Mirzazade S, Shah NJ, Freund HJ. The role of the inferior parietal cortex in linking the tactile perception and manual construction of object shapes. Cereb Cortex. 2001;11:114–121. doi: 10.1093/cercor/11.2.114. [DOI] [PubMed] [Google Scholar]
- Frey SH, Vinton D, Norlund R, Grafton ST. Cortical topography of human anterior intraparietal cortex active during visually-guided grasping. Cogn Brain Res. 2005;23:397–405. doi: 10.1016/j.cogbrainres.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: Command functions for operations within extrapersonal space. J Neurophysiol. 1975;38:871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol. 2000;83:2580–2601. doi: 10.1152/jn.2000.83.5.2580. [DOI] [PubMed] [Google Scholar]
- Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, Wardak C, Durand JB, Vanduffel W. Mapping the parietal cortex of human and non-human primates. Neuropsychologia. 2005;44:2647–2667. doi: 10.1016/j.neuropsychologia.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Pisella L, Grea H, Tilikete C, Vighetto A, Desmurget M, Rode G, Boisson D, Rossetti Y. An ‘automatic pilot’ for the hand in human posterior parietal cortex: toward reinterpreting optic ataxia. Nat Neurosci. 2000;3:729–736. doi: 10.1038/76694. [DOI] [PubMed] [Google Scholar]
- Reina GA, Moran DW, Schwartz AB. On the relationship between joint angular velocity and motor cortical discharge during reaching. J Neurophysiol. 2001;85:2576– 2589. doi: 10.1152/jn.2001.85.6.2576. [DOI] [PubMed] [Google Scholar]
- Rice NJ, Tunik E, Grafton ST. The anterior intrapariietal sulcus mediates grasp execution, independen of requirement to update: New insights from TMS. J Neurosci. 2006 doi: 10.1523/JNEUROSCI.1641-06.2006. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Matelli M. Two different streams form the dorsal visual system: anatomy and functions. Exp Brain Res. 2003;153:146–157. doi: 10.1007/s00221-003-1588-0. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Krams M, Passingham RE. The attentional role of the left parietal cortex: the distinct lateralization and localization of motor attention in the human brain. J Cogn Neurosci. 2001;13:698–710. doi: 10.1162/089892901750363244. [DOI] [PubMed] [Google Scholar]
- Sakata H, Taira M, Mine S, Murata A. Hand-movement related neurons of the posterior parietal cortex of the monkey: their role in visual guidance of hand movements. In: Camaniti R, Johnson PB, Burnod Y, editors. Control of arm movement in space: neurophysiological and computational approaches. Berlin: Springer-Verlag; 1992. pp. 185–198. [Google Scholar]
- Sakata H, Taira M, Murata A, Mine S. Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb Cortex. 1995;5:429–438. doi: 10.1093/cercor/5.5.429. [DOI] [PubMed] [Google Scholar]
- Sakata H, Tsutsui K, Taira M. Toward an understanding of the neural processing for 3D shape perception. Neuropsychologia. 2005;43:151–161. doi: 10.1016/j.neuropsychologia.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Schwartz AB. Cortical neural prosthetics. Annu Rev Neurosci. 2004;27:487–507. doi: 10.1146/annurev.neuro.27.070203.144233. [DOI] [PubMed] [Google Scholar]
- Sergio LE, Hamel-Paquet C, Kalaska JF. Motor cortex neural correlates of output kinematics and kinetics during isometric-force and arm-reaching tasks. J Neurophysiol. 2005;94:2353–2378. doi: 10.1152/jn.00989.2004. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Shikata E, Hamzei F, Glauche V, Knab R, Dettmers C, Weiller C, Buchel C. Surface orientation discrimination activates caudal and anterior intraparietal sulcus in humans: an event-related fMRI study. J Neurophysiol. 2001;85:1309–1314. doi: 10.1152/jn.2001.85.3.1309. [DOI] [PubMed] [Google Scholar]
- Shikata E, Hamzei F, Glauche V, Koch M, Weiller C, Binkofski F, Buchel C. Functional properties and interaction of the anterior and posterior intraparietal areas in humans. Eur J Neurosci. 2003;17:1105–1110. doi: 10.1046/j.1460-9568.2003.02540.x. [DOI] [PubMed] [Google Scholar]
- Shmuelof L, Zohary E. Dissociation between ventral and dorsal fMRI activation during object and action recognition. Neuron. 2005;47:457–470. doi: 10.1016/j.neuron.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Shmuelof L, Zohary E. A mirror representation of others’ actions in the human anterior parietal cortex. J Neurosci. 2006;26:9736–9742. doi: 10.1523/JNEUROSCI.1836-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirigu A, Duhamel JR, Cohen L, Pillon B, Dubois B, Agid Y. The mental representation of hand movements after parietal cortex damage. Science. 1996;273:1564–1568. doi: 10.1126/science.273.5281.1564. [DOI] [PubMed] [Google Scholar]
- Smith AM, Dugas C, Fortier P, Kalaska J, Picard N. Comparing cerebellar and motor cortical activity in reaching and grasping. Can J Neurol Sci. 1993;20(Suppl 3):S53–61. [PubMed] [Google Scholar]
- Taira M, Mine S, Georgopoulos AP, Murata A, Sakata H. Parietal cortex neurons of the monkey related to the visual guidance of hand movement. Exp Brain Res. 1990;83:29–36. doi: 10.1007/BF00232190. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Taira M, Sakata H. Neural mechanisms of three-dimensional vision. Neurosci Res. 2005;51:221–229. doi: 10.1016/j.neures.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Tunik E, Frey SH, Grafton ST. Virtual lesions of the anterior intraparietal area disrupt goal-dependent on-line adjustments of grasp. Nat Neurosci. 2005;8:505–511. doi: 10.1038/nn1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umilta MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, Rizzolatti G. I know what you are doing. a neurophysiological study. Neuron. 2001;31:155–165. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- Vesia M, Monteon JA, Sergio LE, Crawford JD. Hemispheric asymmetry in memory-guided pointing during single-pulse transcranial magnetic stimulation of human parietal cortex. J Neurophysiol. 2006;96:3016–3027. doi: 10.1152/jn.00411.2006. [DOI] [PubMed] [Google Scholar]
- von Economo C, Koskinas GN. Die Cytoarchitektonik der Hirnrinde des Erwachsenen Menschen. Vienna/Berlin: Springerr; 1925. [Google Scholar]
- Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat Neurosci. 2000;3(Suppl):1212–1217. doi: 10.1038/81497. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Goodbody SJ, Husain M. Maintaining internal representations: the role of the human superior parietal lobe. Nat Neurosci. 1998;1:529–533. doi: 10.1038/2245. [DOI] [PubMed] [Google Scholar]


