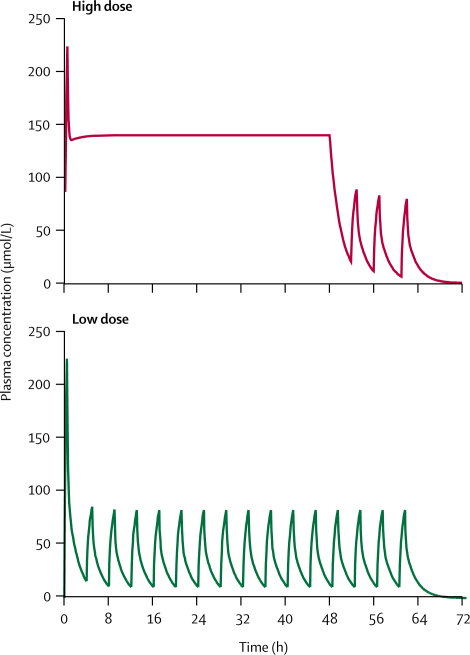
From their randomised trial, Kirti Pawar and colleagues report in today's Lancet on two pralidoxime-dosing schemes in 200 patients who had moderately severe self-poisoning with organophosphorus insecticide.1 After a 2-g loading dose over 30 min, half received a high-dose regimen of 1 g/h pralidoxime iodide for 48 h. The other half received a lower dose: 1 g/h every 4 h.
After 48 h, the lower dose was continued in both groups until the patients could be weaned from the ventilator. The figure shows the expected plasma concentrations of pralidoxime with each regimen.2 Patients who received the high-dose regimen had lower mortality (1% vs 8%) and less intubation and ventilator support, developed less muscle weakness, and required less atropine during the first day, and fewer developed pneumonia.
Figure.
Calculated pralidoxime plasma concentration, two-dose regimen
Calculated for 50-kg person. High dose=2-g bolus over 30 min, then continuous infusion of 1 g/h for 48 h, then 1 g/h every 4 h. Low dose=2-g bolus over 30 min, then 1 g/h every 4 h.
Pawar and colleagues' study is the first known randomised trial that includes large doses of pralidoxime, and suggests that higher doses would be superior to the lower dose (less than 6 g a day) intermittent bolus that is most commonly used in Asia. This region is important because it is where most of the pesticide poisoning in the world takes place and such poisoning accounts for about two-thirds of suicide deaths in the region. These results imply that maintaining higher plasma concentrations of pralidoxime allows the inhibited acetylcholinesterase to be reactivated faster, and provides clinical evidence to support laboratory studies2 showing the oft-cited optimum concentration of 4 mg/L (15 μmol/L) is wildly incorrect.
Pawar and colleagues used pralidoxime iodide. Although the continuous high-dose infusion was well tolerated, an iodine load of about 11·5 g a day is not without risk—the recommended daily intake is just 0·1 mg. Therefore use of pralidoxime chloride or pralidoxime methanesulfonate is preferable, but the dose should account for the different molecular weights of the salts. For example, pralidoxime chloride is 1·53-times more potent than iodide salt. The high-dose regimen of iodide salt is equivalent to 650 mg/h of the chloride salt, similar to the 8 mg/kg per h dose recommended by WHO guidelines.3
Pawar and colleagues' study also challenges another accepted assumption that dimethylated acetylcholinesterase responds poorly to oximes because such drugs do not prevent the dimethyl ester from rapidly ageing (ageing refers to a further chemical reaction of the inhibited enzyme, which completely prevents subsequent reactivation). Two-thirds of the high-dose group had ingested dimethoate, which is more lethal and less responsive to oxime treatment,4 and yet their mortality was low at 1%. But this finding might also be attributable to four favourable conditions in Pawar's study that might not apply elsewhere. First, the time to admission was short (median 2 h) and pralidoxime was given soon after admission. Therefore the early high concentration of pralidoxime could then keep a high proportion of acetylcholinesterase active and theoretically prevent even the dimethyl ester ageing. Second, severely poisoned patients who were not successfully resuscitated in the emergency room were excluded. Third, there was no forced emesis, a procedure that probably results in more harm (from aspiration) than benefit.5 Fourth, Pawar's hospital is well resourced compared with other hospitals in developing countries.
Nevertheless, Pawar and colleagues' study has some major shortcomings that reduce confidence in the results. There were no data to confirm or explain a causal link between the treatment and the outcomes. The response of acetylcholinesterase and neuromuscular function were not measured, nor was the effect of treatment on the pesticide concentration. Crucially, the specific pesticide ingested was not even confirmed. There were also aspects of the trial design that might have inadvertently led to bias.6 For example, the trial was underpowered, there was no blinding, there was a small fixed-block size that could have undermined allocation concealment,7 and there was no reproducible algorithm for atropine dosing or pralidoxime cessation.
These problems might relate to the limited support for clinical research in Asia, especially for independent clinical investigators outside the few centres of excellence. Most future studies on pesticide poisoning will be in such settings in developing countries.8 Some thought should be given as to how best to support more activity, because there is no coordinated international effort to address this problem at the moment, although there are lots of people, organisations, and governments who might be regarded as stakeholders. In a unique procedure supported by The Lancet, the reporting of this study was assisted by two reviewers, who reviewed most of the original data to assist the preparation of a revised manuscript, one of whom travelled on site to discuss critical issues with the authors. How much better would it have been to have this kind of advice before the trial starts?
Pralidoxime is expensive, and high doses might be unaffordable in many places. An affordable pralidoxime preparation should be part of a public-health response to the considerable problem of pesticide poisoning in developing countries.8,9 We believe the drug will save lives, particularly in places where high-tech equipment is not available and many die simply because a respirator cannot be provided for every patient who needs one.10,11 However, this public-health problem would also be helped by better research support for investigators such as Pawar and colleagues, who should be highly commended for their endeavours.
Acknowledgments
We declare that we have no conflict of interest.
References
- 1.Pawar KS, Bhoite RB, Pillay CP, Chavan SC, Malshikare DS, Garad SG. Continuous pralidoxime infusion versus repeated bolus injection to treat organophosphorus pesticide poisoning: a randomised controlled trial. Lancet. 2006;368:2136–2141. doi: 10.1016/S0140-6736(06)69862-0. [DOI] [PubMed] [Google Scholar]
- 2.Eyer P. The role of oximes in the management of organophosphorus pesticide poisoning. Toxicol Rev. 2003;22:165–190. doi: 10.2165/00139709-200322030-00004. [DOI] [PubMed] [Google Scholar]
- 3.Johnson MK, Jacobsen D, Meredith TJ. Evaluation of antidotes for poisoning by organophosphorus pesticides. Emerg Med. 2000;12:22–37. [Google Scholar]
- 4.Eddleston M, Eyer P, Worek F. Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. Lancet. 2005;366:1452–1459. doi: 10.1016/S0140-6736(05)67598-8. [DOI] [PubMed] [Google Scholar]
- 5.European Association of Poisons Centres and Clinical Toxicologists/American Academy of Clinical Toxicology Position paper: ipecac syrup. J Toxicol Clin Toxicol. 2004;42:133–143. [Google Scholar]
- 6.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 7.Schulz KF, Grimes DA. Generation of allocation sequences in randomised trials: chance, not choice. Lancet. 2002;359:515–519. doi: 10.1016/S0140-6736(02)07683-3. [DOI] [PubMed] [Google Scholar]
- 8.Buckley NA, Roberts D, Eddleston M. Overcoming apathy in research on organophosphate poisoning. BMJ. 2004;329:1231–1233. doi: 10.1136/bmj.329.7476.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. QJM. 2000;93:715–731. doi: 10.1093/qjmed/93.11.715. [DOI] [PubMed] [Google Scholar]
- 10.Shivakumar S, Raghavan K, Ishaq RM, Geetha S. Organophosphorus poisoning: a study on the effectiveness of therapy with oximes. J Assoc Phys India. 2006;54:250–251. [PubMed] [Google Scholar]
- 11.Eddleston M, Mohamed F, Davies JO. Respiratory failure in acute organophosphorus pesticide self-poisoning. QJM. 2006;99:513–522. doi: 10.1093/qjmed/hcl065. [DOI] [PMC free article] [PubMed] [Google Scholar]