Abstract
The present research sought to test whether caffeine functioned as a Pavlovian cue in two ways—as a positive drug feature or as a conditional stimulus (CS). As a positive feature (Experiment 1), brief light presentations were followed by sucrose only on sessions in which caffeine (10 mg/kg) was administered. On intermixed saline sessions, light presentations were not followed by sucrose. The light came to control robust goal-tracking (i.e., conditioned responding) only in caffeine sessions. Thus, caffeine disambiguates when the light was paired with sucrose. Decreasing the dose of caffeine decreased the conditioned responding evoked by the light (ED50=4.16 mg/kg). Neither nicotine nor amphetamine substituted for the caffeine feature. As a CS, caffeine (10 or 30 mg/kg, Experiments 2a and 2b, respectively) signaled intermittent access to sucrose—no light presentations. No sucrose or lights were presented on intermixed saline sessions. The caffeine CS, regardless of training dose, acquired the ability to evoke only a weak goal-tracking CR. The nature of this dissociation between caffeine as a drug feature versus a CS is discussed within the context of past research finding a similar dissociation with amphetamine and chlordiazepoxide, but not with nicotine.
Keywords: adenosine, acetylcholine, classical conditioning, discriminative stimulus, dopamine, drug discrimination, modulator, negative feature, occasion setter, reward learning
1. Introduction
Caffeine is one of the more commonly consumed psychoactive substances worldwide and is present in many beverages such as coffee, tea, and soda (Barone and Roberts, 1984) as well as energy drinks. The positively reinforcing effects of caffeine include enhanced wakefulness, increased concentration, and stimulated activity (Daly and Fredholm, 1998; Griffiths et al., 1990; Griffiths and Woodson, 1988). Importantly, the pharmacological effects of caffeine also include an interoceptive stimulus that human and non-human animals can use to discriminate between response options. For example, participants took daily capsules that contained either caffeine or placebo (Griffiths et al., 1990; Oliveto et al., 1992). Based on self-reported drug effects, the participants were able to identify which capsule they had received on a given day. The stimulus effects of caffeine have also been studied in a two-lever operant drug discrimination task with rats. In this task, the pharmacological effects of caffeine indicate that responding on one lever (e.g., right) will be reinforced; responding on the left lever will have no scheduled result. In intermixed vehicle (no caffeine) sessions the response-reinforcer contingency is switched such that left lever presses are now reinforced and right presses are under extinction (Holtzman, 1986, 1987; Mariathasan and Stolerman, 1992; Modrow et al., 1981; Mumford and Holtzman, 1991; Powell et al., 1999).
The pharmacological effects of a drug can also serve as an interoceptive stimulus in Pavlovian (classical) conditioning tasks. As a conditional stimulus (CS), the drug state directly signals the occurrence (or absence) of a biologically and motivationally relevant event (i.e., unconditioned stimulus or US). As a drug feature (i.e., occasion setter), the drug state signals whether or not a CS (e.g., illumination of a light) will be paired with a US. In an array of appetitive and aversive conditioning protocols, such drugs as amphetamine, diazepam, nicotine, ethanol, cocaine, midazolam, morphine, methadone, pentobarbital, and chlordiazepoxide function as a CS and/or a drug feature (e.g., Alessi et al., 2002; Besheer et al., 2004; Bormann and Overton, 1996; Greeley et al., 1984; Jaeger and Mucha, 1990; Maes and Vossen, 1997; Murray and Bevins, in press; Palmatier et al., 2004, 2005; Parker et al., 1994; Revusky et al., 1982; Sokolowska et al., 2002; Troisi and Akins, 2004; Wilkinson et al., 2006).
To our knowledge, there has not been a published report on whether caffeine can serve as an interoceptive stimulus in Pavlovian discrimination tasks. Studying the interoceptive stimulus effects of a drug using an approach informed by Pavlovian conditioning theory and research will add to our understanding of drug states in several important ways. First, such an approach prompts a different set of research questions than those prompted by an operant conditioning approach. Such questions include those concerned about the nature of the conditioned associations between stimuli, as well as the possibility that the later functional impact is altered by the type conditioned associations engendered by different Pavlovian drug discrimination protocols (see Bevins & Palmatier, 2004; Bevins et al., 2006; Palmatier & Bevins, 2007). Second, recent research with nicotine trained as a CS suggests that the neuropharmacological process of a nicotine CS might differ from those of a nicotine discriminative stimulus (Murray & Bevins, in press). If these dissociations hold in future research, then our understanding of the contribution of the interoceptive (i.e., subjective) stimulus effects of drug states to drug abuse liability will require a comprehensive approach.
With these issues in mind, the primary goal of the present research was to assess whether caffeine functioned as a CS and/or a positive drug feature in a Pavlovian appetitive conditioning task recently developed in our laboratory. Published research demonstrating that drug states can serve as a CS for sucrose has primarily focused on nicotine. In these studies, rats received intermixed nicotine and saline sessions. On nicotine sessions, sucrose was intermittently available; no sucrose was available on saline sessions. Using anticipatory head entries into the sucrose receptacle as a measure of conditioned responding (i.e., goal-tracking; Boakes, 1977; Farwell and Ayres, 1979), we found an increase in goal-tracking on nicotine compared to saline sessions (Besheer et al., 2004; Bevins and Palmatier, 2004; Bevins et al., in press; Murray and Bevins, in press; Wilkinson et al., 2006). For the present research we used procedures shown to be effective with nicotine (Besheer et al., 2004) to assess whether caffeine (10 or 30 mg/kg) could function as an appetitive interoceptive CS in this Pavlovian drug discrimination task.
In studies examining positive drug features, the drug state indicated that presentation of a discrete CS (e.g., 15-s presentation of a light or white noise) was followed by brief access to sucrose. On intermixed saline sessions, the same discrete CS was presented but was not followed by sucrose. In other words, the drug state occasioned when CS presentations were reinforced. Conditional control of goal-tracking was demonstrated by a differential increase in dipper entries during CS presentations only in the drug state (Palmatier et al., 2004, 2005; Palmatier and Bevins, 2007). For the present research, we used these procedures shown to be effective with nicotine, amphetamine, and chlordiazepoxide to assess whether caffeine could function as a positive feature for an appetitive CS-US relation. Notably, this training protocol is similar to the CS training protocol except that in the feature positive experiment discrete light CSs are presented (see Materials and methods).
In the present research, caffeine readily functioned as a positive drug feature. Accordingly, a secondary goal of the present study was to describe the caffeine generalization function and determine the specificity of the discrimination by testing whether nicotine or amphetamine could substitute for the caffeine feature. That is, the discrimination might not be specific to the pharmacological properties of caffeine. Rather, differential control of conditioned responding might reflect a drug versus no drug or a stimulant versus non-stimulant discrimination. The substitution pattern by nicotine and amphetamine would allow us to evaluate these possibilities.
2. Materials and methods
2.1. Subjects
Male Sprague-Dawley rats (370±7 g at start of study) were obtained from Harlan (Indianapolis, Indiana, USA). Rats were housed individually in clear 48.3 × 26.7 × 20.3 cm (l × w × h) polycarbonate tubs lined with aspen shavings in a colony that was temperature and humidity controlled. All sessions were conducted during the light portion of a 12 h light:dark cycle. Water was continuously available in the home cage. Food (Harlan Teklad Rodent Diet) access was restricted such that rats were kept at 85% of free-feeding body weights. Approximately every 4 weeks this target 85% weight was increased by 2 g. Protocols were approved by the University of Nebraska-Lincoln Animal Care and Use Committee and followed the ‘Guide for the Care and Use of Laboratory Animals’ (National Research Council, 1996).
2.2. Apparatus
Seven conditioning chambers (ENV-008CT; Med Associates, Inc., Georgia, VT, USA) measuring 30.5 × 24.1 × 21 cm (l × w × h) were used throughout the study. Sidewalls were aluminum; the ceiling, and front and back walls were clear polycarbonate. Chambers were equipped with two white cue lights (2.54 cm dia; 28 V, 100 mA) mounted on one sidewall, 14.6 cm above the metal rod floor and 3.5 cm from the front and back walls. Illumination of the lights served as the discrete cue in the positive feature experiment. A recessed receptacle (5.2 × 5.2 × 3.8 cm; l × w × d) was centered between the lights on the same sidewall. The bottom of the receptacle was 13 cm below the center of the lights. A dipper arm raised a 0.1-ml cup of 26% sucrose solution (w/v) into the receptacle. An infrared emitter/detector unit mounted 1.2 cm into the receptacle and 3 cm from the floor monitored head entries into the dipper. A second infrared emitter/detector unit positioned 4 cm above the rod floor bisected the chamber 14.5 cm from the sidewall containing the receptacle and lights. The number of times this beam was broken provided a measure of general chamber activity during generalization and substitution testing (see later). Each chamber was enclosed in a light and sound attenuating cubicle fitted with a fan to provide airflow and mask noise. A personal computer with Med Associates, Inc. interface and software (Med-PC for Windows, version IV) controlled stimulus events and recorded dipper entries and chamber activity.
2.3. Drugs
Caffeine anhydrous, (−)-nicotine hydrogen tartrate, and d-amphetamine sulfate were purchased from Sigma (St. Louis, MO, USA). Drugs were mixed in 0.9% saline solution (w/v). Nicotine was adjusted to a pH of 7.0±0.2 using a dilute NaOH solution. All solutions in the positive feature study (Experiment 1) were injected at 1 ml/kg except for 30 mg/kg caffeine which was injected at 2 ml/kg during generalization testing. In the CS experiments, caffeine was injected at 2 ml/kg. Caffeine and amphetamine were injected intraperitoneally (IP) 15 min before placement into the chamber; nicotine was injected subcutaneously (SC) 5 min before placement. Nicotine doses are reported in the base form; remaining drug doses are reported in salt form. Drug doses, routes of administration, and injection-to-placement intervals were based on past research from our laboratory and others (Besheer et al., 2004; Holtzman, 1986; Mariathasan and Stolerman, 1992; Palmatier et al., 2004, 2005).
2.4. Experiment 1: Caffeine as a Positive Feature
2.4.1. Acquisition
Rats (n=6) that had previously been used in a brief drug-free novelty task were handled for 3 min per day for 3 days before the start of the present experiment. Rats were injected with 10 mg/kg caffeine or saline 15 min before chamber placement. During each 20-min session, there were 8 presentations of the 15-s light CS. On caffeine sessions, each offset of the stimulus lights was followed immediately by 4-s access to sucrose. To decrease predictability of the light stimuli within and between sessions, four different computer programs controlling lights and sucrose deliveries were used. The average time to the first light onset was 135 s (range of 90–180 s) and the average intertrial interval was 120 s (range of 75–165 s). For saline sessions, light presentations were matched with those of caffeine sessions and 4-s ‘empty’ intervals occurred in place of sucrose to insure identical session length. Caffeine and saline sessions were intermixed randomly with the restriction that no more than two of a session type (caffeine or saline) occurred in a row and that all four programs for each session type were used across eight session blocks. Training continued for 22 caffeine and 22 saline sessions.
2.4.2. Generalization Testing
Following acquisition of the discrimination, rats were tested for generalization to varying doses of caffeine (0.625, 1.25, 2.5, 5, 10, 20, and 30 mg/kg), nicotine (0.025, 0.05, 0.1, and 0.2 mg/kg), and amphetamine (0.125, 0.25, 0.5, and 1 mg/kg). On the first 4 consecutive days of a 5-day cycle, rats received two caffeine and two saline training sessions intermixed as described for acquisition training. If a rat met the discrimination criteria (see later), a 4-min test session was conducted on day 5 of the cycle. During each test, there was a single presentation of the light CS. Four different test programs, presented in random order, matched the timing of the first light presentation to the four training programs. If discrimination criteria for testing were not met, the rat remained in its home cage on day 5 and resumed training on the following day. Importantly, these 5-day cycles continued until each rat completed testing with all ligands and doses unless otherwise noted.
Testing of caffeine and nicotine was intermixed. Each dose was tested in two different random orders. Nicotine doses above 0.2 mg/kg were not tested because acute injections of these higher doses consistently produce locomotor ataxia (e.g., Bevins et al., 2001). Upon completion of the caffeine and nicotine generalization tests, rats were tested with two different random orders of amphetamine. On six occasions, additional tests were conducted due to discrepant findings on the initial two tests with the same dose/compound. For each rat, dipper entries were averaged over repeated tests and these averages were used for analyses and figures. Additionally, two saline test sessions were intermixed within the testing orders to provide a baseline for comparison. One saline injection was IP 15 min before placement in the chamber (cf. caffeine and amphetamine injections); the other saline injection was SC 5 min before placement (cf. nicotine injections). The order of these injection types was random for each rat. Given the lack of statistical difference between these saline tests, t(5)=1.41, P=0.217, the mean for the two test sessions was used for analyses and figures.
2.4.3. Dependent Measures and Criteria
The primary dependent measure for acquisition and test sessions was an elevation score: the number of dipper entries recorded during the 15-s presentation of the light CS minus the number of dipper entries recorded during the 15-s interval before the light (CS period – pre-CS period). A positive value indicates more dipper entries during the CS. Additionally, a rate measure was calculated by subtracting the number of dipper entries per second during the CS from the number of dipper entries per second during the pre-CS period (cf. Experiment 2a and 2b). Before each generalization test was conducted, rats were required to meet performance criteria based on the elevation scores calculated for the first 4 drug and saline sessions of each 5-day cycle. On average, elevation scores on drug sessions had to be at least three responses greater than elevation scores on respective saline session (1st drug session vs. 1st and 2nd saline session, etc.). Additionally, on the last caffeine session, the elevation score for the first light presentation had to be at least one higher than the last saline session (cf. Bevins et al., 2006; Palmatier et al., 2004, 2005). General activity was defined as number of chamber beam breaks in the 4-min test sessions.
2.5. Experiment 2a: 10 mg/kg Caffeine as a CS
2.5.1. Preliminary Training
Rats (n=6) were previously used in either a brief place conditioning or locomotor conditioning study before the start of the present experiment. Rats were handled for at least 3 min per day for 3 days before dipper training commenced. Dipper training consisted of three 50-min sessions. Each daily session was initiated with the rat’s first head entry into the dipper. The probability of receiving sucrose decreased from 0.167 to 0.05 per 60 s over the three sessions (approximately 2.5 to 0.75 sucrose deliveries per min).
2.5.2. Acquisition
Acquisition began the day after the last dipper training session and was virtually identical to the positive feature procedures of Experiment 1 except the light cues were not presented in any session. During caffeine sessions, the time varied between sucrose deliveries (mean = 141 s; range 90–210 s) and the first sucrose presentation (mean = 120 s; range 90–150 s). On saline sessions, no sucrose was delivered, but programs were matched to the timing of caffeine programs with a 4-s ‘empty’ interval where sucrose would have been delivered to equate the dipper entry data for later analysis. Training continued for 28 caffeine and 28 saline sessions. Conditioned responding evoked by the caffeine CS was weak and did not lend itself to generalization and substitution testing (see Results). Thus, training was longer than the positive feature experiment in an attempt to enhance conditioned response strength.
2.5.4. Dependent Measure
An elevation score cannot be calculated in Experiment 2a (or Experiment 2b; see later) given that the CS is the interoceptive effects of caffeine and not brief illumination of cue lights. Accordingly, the primary dependent measure for acquisition was the rate of dipper entries per s before the first sucrose delivery or an equivalent time on saline sessions. Using only dipper entries before the first sucrose delivery avoids any influence of the US in the measure of conditioning. Further, a rate measure was used because time to the first sucrose delivery varied across sessions (cf. Besheer et al., 2004; Murray and Bevins, in press; Wilkinson et al., 2006).
2.6. Experiment 2b: 30 mg/kg Caffeine as a CS
Rats (n=7) that served previously in a brief place conditioning or locomotor conditioning procedure were used in this experiment. Handling, dipper training, acquisition, and the dependent measure were identical to Experiment 2a except that 30 mg/kg caffeine was used as the CS.
2.7. Data Analyses
Two-way ANOVAs were conducted on elevation scores (Experiment 1) and dipper entry rates (Experiments 2a and 2b) across caffeine versus saline sessions. Significant interactions prompted Fisher’s least significant difference (LSD) tests that control for Type I error rate. For generalization and substitution testing, one-way ANOVAs determined if there were effects of test doses. General chamber activity was analyzed in a similar fashion. A significant F-value prompted Fisher’s LSD tests to compare test doses of drug to a saline baseline, as well as the training dose of caffeine. A median effective dose (ED50) was calculated using the ascending limb of the caffeine generalization curve. Statistical significance was declared using a two-tailed rejection region of 0.05 for all tests.
3. Results
3.1. Experiment 1: Caffeine as a Positive Feature
3.1.1. Acquisition
Figure 1A shows the average elevation score of the eight light CS presentations per session across acquisition training. A two-way repeated measures ANOVA revealed a main effect of Drug (caffeine versus saline), F(1,5)=64.93, P<0.001, a main effect of Session, F(21,105)=9.20, P<0.001, and a significant Drug x Session interaction, F(21,105)=8.34, P<0.001, Mean square error (MSE)=1.29. Elevation scores on caffeine sessions were higher than recorded on saline sessions 12 to 22, LSDminimum mean difference (mmd)=1.31. Figure 1B shows the elevation score for the first light presentation of each session across acquisition. For the elevation score measure, there was a main effect of Drug, F(1,5)=11.03, P=0.021, a main effect of Session, F(21,105)=2.31, P=0.003, and a significant Drug x Session interaction, F(21,105)=2.36, P=0.002, MSE=4.75. The first elevation scores on caffeine sessions were higher than on saline sessions 17 to 22, LSDmmd=2.52. For comparison with the CS data of Experiments 2a and 2b, the right y-axis shows the elevation score based on the rate of dipper entries. Obviously, the results of the ANOVA were identical since this measure merely divides all pre-CS and CS values by 15 s; the LSDmmd was 0.167 (MSE=0.021).
Figure 1.
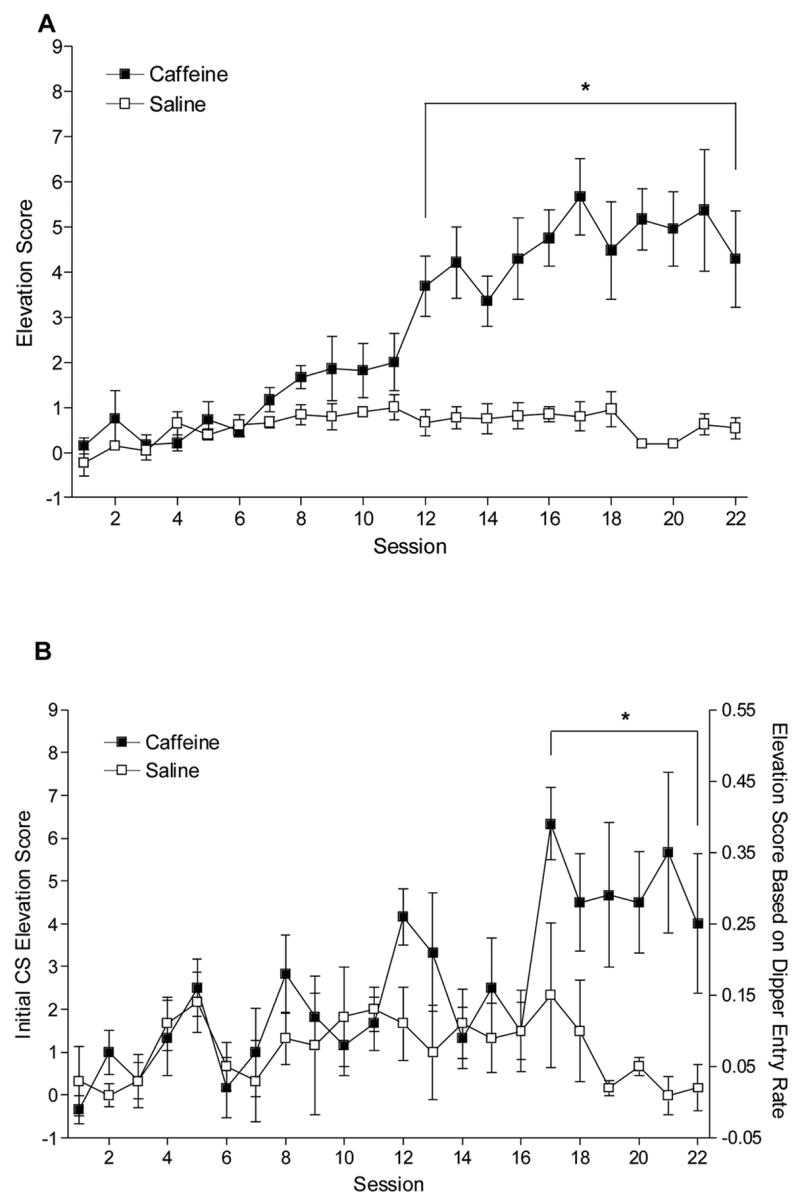
Panel A shows the mean elevation scores (±1 SEM) for acquisition of the caffeine positive feature in Experiment 1. Panel B shows acquisition using the first elevation scores. The left axis shows elevation scores, and the right axis shows the same elevation score based on dipper entries per s (±1 SEM). For both panels, * indicates a significant difference between the corresponding caffeine and saline session, P<0.05.
3.1.2. Generalization and Substitution Testing
For the generalization function for caffeine as a positive feature (see Figure 2A) there was a significant effect of Dose, F(7,35)=4.78, P=0.001, MSE=6.19. Elevation scores were higher on 5 to 30 mg/kg caffeine than on saline, LSD mmd=2.93. Additionally, elevation scores were lower than the caffeine training dose (10 mg/kg) at 0 to 2.5 mg/kg caffeine. The ED50 for the linear portion of the caffeine generalization curve was 4.16 mg/kg. Figure 2B shows general chamber activity during the caffeine generalization tests. The one-way ANOVA revealed that activity differed across caffeine dose, F(7,35)=2.95, P=0.015, MSE=426.22. Activity was higher on 1.25 to 20 mg/kg caffeine than saline, LSD mmd=24.34. Conversely, relative to the caffeine training dose, activity was significantly lower for 0.625 and 30 mg/kg and saline.
Figure 2.
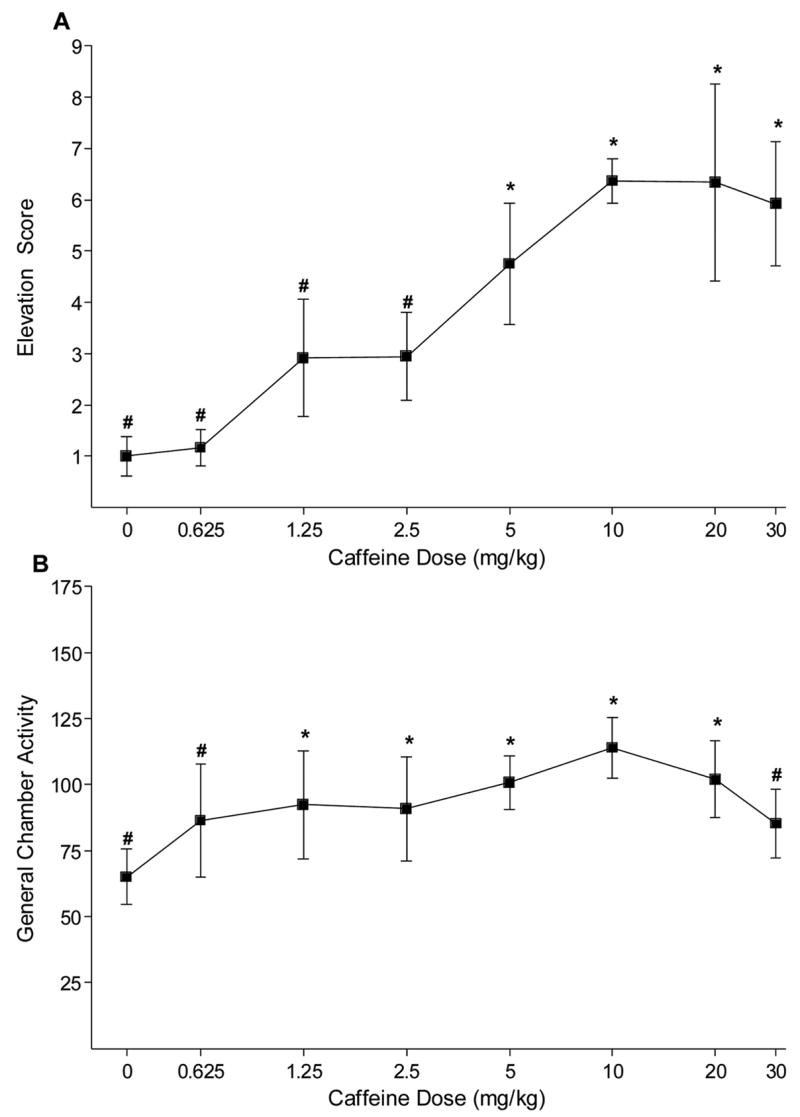
Panel A shows the mean elevation scores (±1 SEM) on caffeine generalization tests of Experiment 1. Panel B shows the mean general activity (±1 SEM). For both panels, * indicates a significant difference from saline. # represents a significant difference from the caffeine training dose (10 mg/kg), P<0.05.
Nicotine did not substitute for the caffeine positive feature, F(4,20)=2.34, P=0.09 (Figure 3A). Further, nicotine did not significantly alter general chamber activity during nicotine substitution testing, F(4,20)=2.40, P=0.084 (Figure 3B). Figure 3C shows the amphetamine substitution results. One rat consistently failed to meet discrimination criteria in this test phase and was removed during this portion of the study. Amphetamine did not substitute for the caffeine positive feature, F(4,16)=2.34, P=0.099. However, amphetamine did alter general chamber activity, F(4,16)=4.47, P=0.013, MSE=502.32 (Figure 3D). Activity was higher on 0.25 to 1 mg/kg amphetamine than saline, LSD mmd=30.05. For comparison, an activity baseline for caffeine was generated from the chamber beam breaks in the first 4 min of the last caffeine training session before testing 0.5 mg/kg amphetamine. Because each rat was tested twice on amphetamine, this value was randomly taken from the first or second test session for a given rat (Mean Activity=104±4 beam breaks; see solid line with juxtaposed dashed lines in Figure 3D). Amphetamine-induced activity was not significantly different from this caffeine baseline.
Figure 3.
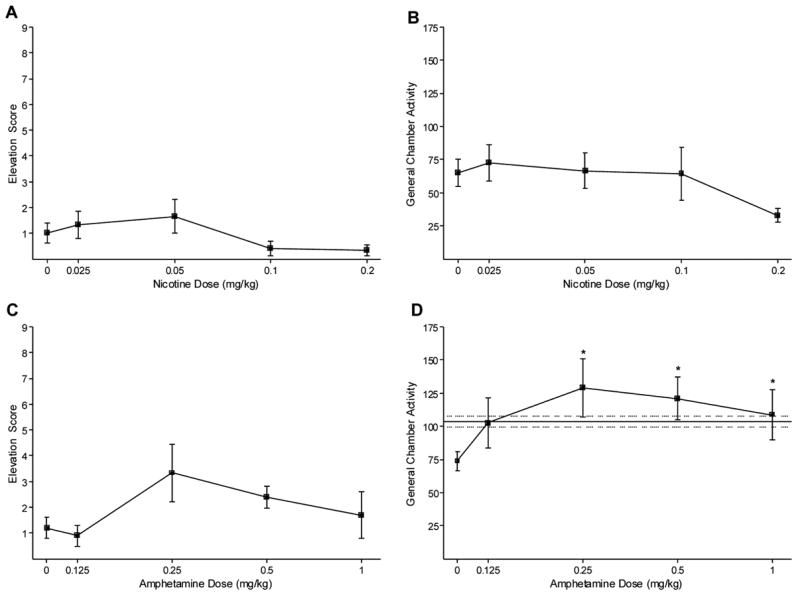
Panel A shows the mean elevation scores (±1 SEM) of nicotine substitution in Experiment 1. Panel B shows the mean general activity (±1 SEM) of nicotine substitution. Panel C shows the mean elevation scores (±1 SEM) of amphetamine substitution. Panel D shows the mean general activity (±1 SEM) of amphetamine substitution. * indicates a significant difference from saline. The solid line indicates the baseline activity of the caffeine training dose (dotted lines ±1 SEM), P<0.05.
3.2. Experiment 2a: 10 mg/kg Caffeine as a CS
For acquisition training with the 10 mg/kg caffeine CS, there was a main effect of Session, F(27, 135)=9.74, P<0.001, and a significant Drug x Session interaction, F(27,135)=1.78, P=0.017, MSE=0.001; the main effect of Drug, F(1,5)=4.67, P=0.08, was not significant (see Figure 4A). Follow-up analyses indicated that dipper entry rates were lower on caffeine than saline for session 2, and higher on caffeine than saline for sessions 8, 11–12, 16–18, and 21–28, LSDmmd=0.04. These results indicate that, albeit weak, caffeine acquired control of goal tracking.
Figure 4.
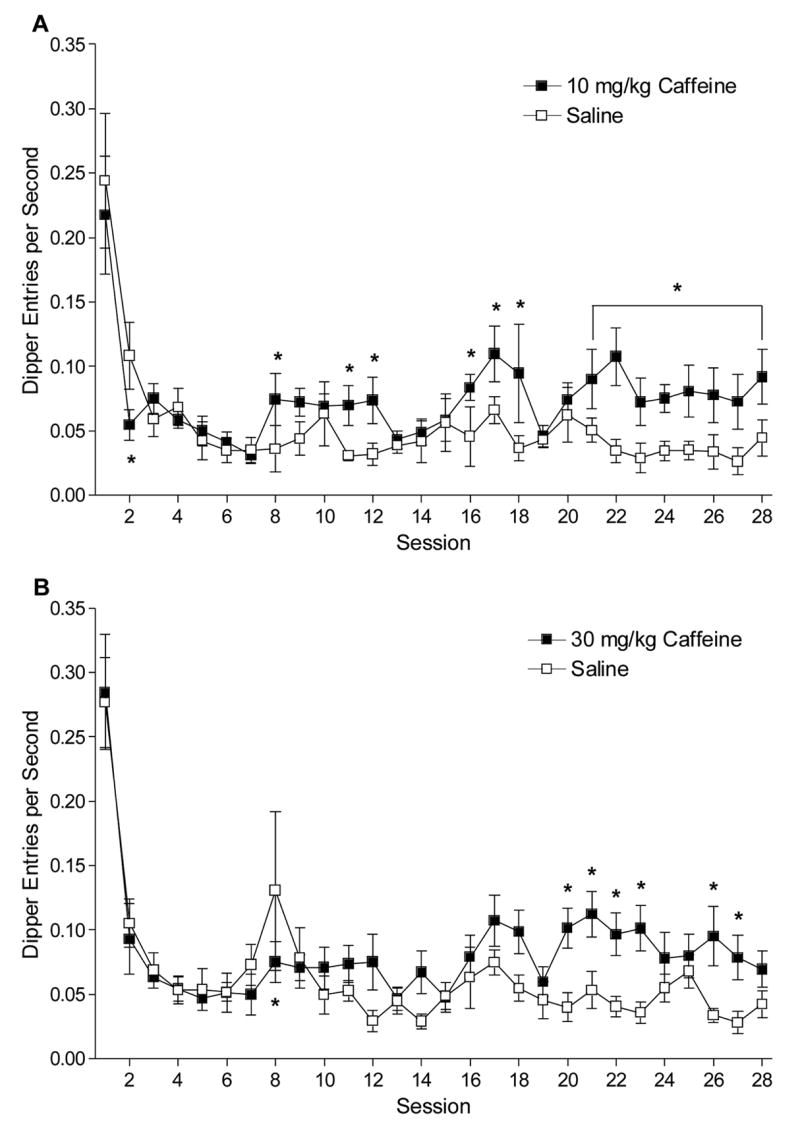
Panel A shows the dipper entry rates (±1 SEM) for acquisition of the 10 mg/kg caffeine CS (Experiment 2a). Panel B shows the dipper entry rates (±1 SEM) for acquisition of the 30 mg/kg caffeine CS (Experiment 2b). For a rat in this experiment, 2 caffeine sessions were given that should have been saline sessions. These 2 caffeine sessions were not included in analysis, but to prevent loss of all data in the repeated measures ANOVA, saline values were generated by averaging the saline responding of the sessions before and after the missed saline sessions. For both panels, * indicates a significant difference between corresponding caffeine and saline session, P<0.05.
3.3. Experiment 2b: 30 mg/kg Caffeine as a CS
For acquisition training with the 30 mg/kg caffeine CS, there was a main effect of Drug, F(1,6)=11.27, P=0.015, a main effect of Session, F(27,162)=11.50, P<0.001, and a significant Drug x Session interaction, F(27,162)=1.61, P<0.036, MSE=0.002 (see Figure 4B). Conditioned responding was lower on caffeine than saline for session 8, but higher on sessions 20–23 and 26–27, LSDmmd=0.05. This pattern of results also indicates weak and transient control of a goal-tracking CR by a higher dose of caffeine.
4. General Discussion
Caffeine readily functioned as a positive feature (occasion setter) in this appetitive Pavlovian drug conditioning task. That is, anticipatory head entries into the dipper increased during the light CS only on caffeine sessions; no such increase during the same light was seen on saline sessions. This finding extends past research by adding caffeine to the list of drugs that function as a positive feature including amphetamine, nicotine, cocaine, midazolam, morphine, methadone, pentobarbital, and chlordiazepoxide (Jaeger and Mucha, 1990; Maes and Vossen, 1997; Palmatier et al., 2005; Parker et al., 1994; Revusky et al., 1982; Troisi and Akins, 2004). Notably, when we used an average of the 8 trials within each training session as the dependent measure (see Figure 1A), conditioned responding emerged after 24 sessions (12 caffeine and 12 saline sessions intermixed). This measure may, however, over estimate acquisition speed of the drug discrimination. That is, sucrose delivery after the initial light presentation, rather than caffeine, might function as a signal for later deliveries of sucrose on caffeine sessions. Using only the first light presentation as the measure of conditioned responding avoids this difficulty. With this measure, conditioned responding to the light CS, which must be ‘modulated’ by the interoceptive effects of caffeine, required 34 sessions to emerge (17 of each session type). In the present research, the acquisition rate using 10 mg/kg caffeine as the drug feature was similar to the two-lever operant drug discrimination paradigm with food reinforcer using higher caffeine doses. For example, Mariathasan and Stolerman (1992) found that rats took an average of 40 sessions to discriminate 20 mg/kg caffeine from saline. Modrow et al. (1980) reported acquisition within approximately 50 sessions if 32 mg/kg caffeine was the training dose. When comparable caffeine doses were used in a discrete shock avoidance task, 10 mg/kg caffeine took between 51 and 108 sessions to acquire control of responding (Holtzman, 1986, 1987; Mumford and Holtzman, 1991; Powell et al., 1999).
Conditional control of responding to the light CS was sensitive to the dose of caffeine during testing. Rats trained with caffeine as a positive feature showed a decrease in conditioned responding to the light as the test dose decreased below the 10 mg/kg training dose; increasing the dose up to 30 mg/kg had little effect on the CR (see Holtzman, 1986; Mumford and Holtzman, 1991; Powell et al., 1999 for a similar pattern with a caffeine discriminative stimulus). The ED50 for the caffeine feature was 4.16 mg/kg. This value is similar to that in the operant drug discrimination literature (Holtzman 1986, training dose=10 mg/kg, ED50=3.17 mg/kg).
Nicotine did not substitute for caffeine in the present study suggesting that nicotinic acetylcholine receptors do not play a role in the interoceptive stimulus effects of 10 mg/kg caffeine. This is somewhat in contrast to the findings of Palmatier et al. (2005) in which caffeine substituted for a nicotine positive feature. First, the interoceptive caffeine stimulus might differ when high versus low doses of the drug are administered (e.g., Mumford and Holtzman, 1991). For example, when caffeine substituted for 0.4 mg base/kg nicotine (Palmatier et al., 2005) the ED50 was higher than the 10 mg/kg training dose used in Experiment 1 (i.e., ED50=15.45 mg/kg). Thus, higher doses of caffeine and nicotine may share similar stimulus properties, whereas lower doses are more dissociable. Unfortunately, nicotine doses above 0.2 mg/kg in the present research would have likely produced severe locomotor impairment when given acutely, precluding any meaningful test of these high doses. A second reason that these findings contrast with those of Palmatier et al. (2005) is that substitution between the stimulus effects of nicotine and caffeine may be asymmetrical. The failure of Modrow et al. (1981) to find substitution of nicotine (0.1 to 0.4 mg/kg) for a relatively high dose (32 mg/kg) caffeine discriminative stimulus supports this asymmetry account. Further studies are needed to help decide between these possible explanations.
Amphetamine did not substitute for the caffeine feature in Experiment 1. Although the mean level of dipper entries during the light CS increased at the 0.25 mg/kg amphetamine dose, this trend did not continue at higher doses and there was not a significant F-value on this substitution data. Accordingly, the result suggests that this low dose of caffeine does not differentially modulate conditioned responding to a light CS via increased synaptic dopamine. Interestingly, in the operant drug discrimination literature, amphetamine partially to fully substitutes for the discriminative stimulus effects of caffeine. For example, in the discrete shock avoidance paradigm, 1 mg/kg amphetamine fully substituted for a 10 mg/kg caffeine discriminative stimulus (Mumford and Holtzman, 1991). In the appetitive paradigm, the 1 mg/kg dose of amphetamine fully substituted for a 20 mg/kg caffeine discriminative stimulus (Mariathasan and Stolerman, 1992); 2 mg/kg amphetamine partially substituted for a 32 mg/kg caffeine discriminative stimulus (Modrow et al., 1981). This difference between the caffeine as a positive drug feature in a Pavlovian goal-tracking task and the schedule-controlled two-lever operant discrimination task highlights the possibility that the neuropharmacological processes underlying the signaling effects of caffeine may differ depending on its behavioral function (see Palmatier et al., 2005 for a similar suggestion). As such, it will be important to explore the potential similarities and differences. One especially interesting avenue for this research will be in the transfer of a discriminative stimulus for a positive feature and vice versa (cf. Davidson et al., 1988). Will transfer occur and does a drug’s training history as a discriminative stimulus or positive feature affect the substitution and antagonism pattern of other ligands?
The lack of nicotine or amphetamine substitution for the caffeine feature indicates that discrimination performance was maintained by more than the presence or absence of a drug. That is, there was pharmacological specificity to the interoceptive stimulus effects of the caffeine in the present positive drug feature task. Further, the lack of amphetamine substitution, a classic locomotor stimulant (e.g., Geyer et al., 1986), suggests that the discrimination was based on more than whether the drug has or does not have stimulant effects. In fact, amphetamine (0.25 to 1 mg/kg) significantly increased chamber activity above that of saline, yet that activity was comparable to caffeine (cf. Figure 3D). However, this enhanced locomotion did not translate into increased dipper entries during the light CS on substitution tests. This pattern of results clearly eliminates a psychomotor stimulant account of caffeine’s modulatory control over goal-tracking and further supports the potential utility of the Pavlovian drug discrimination task for studying the interoceptive stimulus effects of drugs.
In Experiment 2a, caffeine at 10 mg/kg functioned, at best, as a weak interoceptive contextual CS. As a contextual CS, caffeine signaled that sucrose would be available intermittently throughout the session. This weak control of a goal-tracking CR is in contrast to the ability of caffeine at 10 mg/kg to function as a Pavlovian drug feature (Experiment 1) and as an operant discriminative stimulus (e.g., Mumford and Holtzman, 1991). This difference is remarkable given the similarity of the procedures used in each of the current experiments. At least three possible accounts of this difference can be addressed by the present research. One possibility is that the salience of the caffeine was not sufficient for it to function as a contextual CS. This account is made less tenable by the fact that caffeine at 10 mg/kg readily served as a contextual occasion setter (Experiment 1). However, as an occasion setter, weak stimulus salience might be aided by the presentation of a brief light CS. Additionally, when the caffeine dose was increased to 30 mg/kg (Experiment 2b) the drug stimulus did not control robust goal tracking. In fact, goal tracking at this higher dose appeared to be worse.
A second alternative account would suggest that the rats fail to learn the discrimination because without a discrete CS to signal sucrose, the rats fail to obtain the sucrose in these caffeine CS sessions (cf. drug feature protocol). We are confident that the rats received most of the sucrose because we had written into the computer programs code that counted dipper entries during each 4-s sucrose delivery. During the last five caffeine sessions, the 10 mg/kg group (Experiment 2a) had dipper entries during 79% of deliveries; the 30 mg/kg group (Experiment 2b) had dipper entries during 84% of deliveries.
Finally, the difference in preliminary training between the two tasks may account for the current findings. That is, rats in the CS experiments (Experiments 2a and 2b) were trained to access sucrose before beginning acquisition; rats in the drug feature experiment (Experiment 1) were not. Our enthusiasm for this explanation is severely diminished by recently published research from our laboratory (Palmatier and Bevins, 2007). In that research, all rats were given preliminary dipper training as described for the present caffeine CS experiments using amphetamine or chlordiazepoxide as the interoceptive Pavlovian stimulus. Similar to the findings in the current research, the amphetamine and chlordiazepoxide readily functioned as positive drug features, yet the same drugs in the CS task did not (Palmatier and Bevins, 2007). Further, nicotine readily functions as a CS in rats that have received preliminary dipper training (Besheer et al., 2004) indicating that dipper training before excitatory conditioning of a drug state CS does not prevent acquisition of a CR. Combined, these findings suggest that we look elsewhere for a possible explanation for the differences in caffeine as a CS versus drug feature.
Although nicotine readily functions as a CS and evokes a robust and stable CR under the current training protocol (Besheer et al., 2004), the poor control of conditioned responding by the caffeine CS is not completely surprising. We have found that amphetamine and chlordiazepoxide do not directly evoke a goal-tracking CR even though both drugs readily serve as drug features that facilitate responding to a discrete stimulus (Palmatier et al., 2005; Palmatier and Bevins, 2007). Combined, these findings pose an interesting puzzle regarding why chlordiazepoxide and amphetamine do not readily function as an interoceptive CS and caffeine evokes only a weak conditioned response, yet these same drugs serve as drug features that modulate the same goal-tracking CR to a discrete stimulus. The only substantive procedural difference between the CS and positive feature protocol is the presence of the discrete stimulus occurring immediately before each sucrose delivery. Drug dose, injection-to-placement interval, number of trials per session, etc. are identical. Two, nicotine evokes/modulates a goal-tracking response whether trained as a CS or as a positive drug feature. As noted by Palmatier and Bevins (2007), an account based on drug effects overshadowing the rewarding effects of sucrose predicts that none of the drugs would serve as a feature. That is, if processing of the drug CS is effectively competing with and minimizing processing of the US, then the drugs should also not function as positive features. A similar criticism applies to a response interference account.
This discussion tempts us to conclude that there is something distinct about nicotine that allows it to acquire direct control of a goal-tracking CR. Indeed, there is a body of literature demonstrating that nicotine enhances the incentive salience/unconditioned reinforcing effects of stimuli. For example, nicotine exposure in rats increases Pavlovian discriminative approach behavior for water (Olausson et al., 2003; 2004), as well as operant responding for a mildly reinforcing visual stimulus (Donny et al., 2003). Perhaps such a mechanism can help us explain why nicotine appears to function better as a CS in this Pavlovian appetitive conditioning task than the others drugs tested to date (i.e., amphetamine, caffeine, and chlordiazepoxide). Presently, we assume that a drug state CS should acquire additional appetitive properties by virtue of its pairings with sucrose—apparently these pairing are insufficient except for nicotine to evoke a robust CR. The incentive-enhancing effect of nicotine might increase the appetitive effects of sucrose. If so, such an increase is functionally equivalent to using a better quality US when nicotine serves as the interoceptive Pavlovian stimulus [see Bevins and Palmatier (2004) for a more detailed discussion of this potential interaction]. A general result in Pavlovian conditioning research is that conditioned excitation (CR magnitude) increases with US quality (e.g., Bevins et al., 1997; Pavlov, 1927).
This enhanced incentive salience account makes several predictions that deserve attention. One prediction is that conditioned responding evoked by the discrete CS in positive feature experiments should be higher when nicotine serves as the feature. Our research has not supported this prediction. For example, there was little difference in the expression of conditioned responding when rats were trained with nicotine, chlordiazepoxide, or amphetamine as positive drug features (Palmatier et al., 2005). However, this counter evidence should be taken with some caution. That is, the measure of conditioning is an increase in responding during a 15-s CS. Perhaps dipper entries are at a maximum when a 15 s CS is employed. Such a ceiling effect would obscure our ability to see an increase in CR magnitude with drug state. Also, this account suggests that a more appetitive conditioning situation could work with the other drug states as CSs. Indeed, the 10 mg/kg caffeine CS did evoke a weak CR. Perhaps increasing the number of sucrose deliveries per session would increase the CR magnitude in a manner similar to that shown with a nicotine CS (Wilkinson et al., 2006). Albeit possible, this strategy did not work for amphetamine. As noted earlier, amphetamine did not function as a CS using the 8 sucrose deliveries of present research (Palmatier and Bevins, 2007). A recent unpublished experiment in our laboratory has shown that even 36 sucrose deliveries in 20-min sessions was not sufficient for amphetamine to serve as a CS. Finally, our enthusiasm for the nicotine incentive enhancement account is further diminished by the finding that amphetamine also enhances the incentive effects of stimuli (e.g., Simon and Setlow, 2006).
In sum, the present research extends the stimulus effects of caffeine to the role of facilitating conditioned responding to an appetitive CS-US association. As a positive feature, caffeine’s modulatory control was pharmacologically specific; neither amphetamine nor nicotine substituted for the caffeine feature. In contrast, caffeine’s ability to directly evoke a conditioned response (i.e., function as a CS) was sufficiently weak that it did not allow for subsequent generalization testing. Future research will need to parse apart controlling variables relevant for the interoceptive effects of a drug to serve as a positive feature (occasion setter) versus a conditional stimulus. Given that a nicotine CS readily evokes robust conditioning responding unlike chlordiazepoxide, amphetamine, or caffeine, part of this process might include identifying what makes the interoceptive effects of nicotine distinct in this protocol.
Acknowledgments
We thank Dawn M. Metschke for her assistance with conducting the caffeine CS experiments. We also thank Kevin P. Myers for his thoughtful comments on an earlier version of this report. The research and Rick A. Bevins were supported by United States Public Health Service grants DA018114 and DA11893. MED-PC programs functionally similar to those used in the present article are available upon request. Correspondence related to this article should be addressed to Rick A. Bevins, Department of Psychology, University of Nebraska-Lincoln, Lincoln NE USA 68588-0308, or e-mail rbevins1@unl.edu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessi SM, Roll JM, Reilly MP, Johanson C-E. Establishment of a diazepam preference in human volunteers following a differential-conditioning history of placebo versus diazepam choice. Exp Clin Psychopharmacol. 2002;10:77–83. [PubMed] [Google Scholar]
- Barone JJ, Roberts H. Human consumption of caffeine. In: Dews PB, editor. Caffeine: Perspectives from recent research. Springer-Verlag; Berlin; New York: pp. 59–73. [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, Bevins RA. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology. 2004;172:108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Bevins RA, McPhee JE, Rauhut AS, Ayres JJB. Converging evidence for one-trial context fear conditioning with an immediate shock: Importance of shock potency. J Exp Psych: Anim Behav Process. 1997;23:312–324. doi: 10.1037//0097-7403.23.3.312. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, Pickett KS. Nicotine-conditioned locomotor activity in rats: dopaminergic and GABAergic influences on conditioned expression. Pharmacol Biochem Behav. 2001;68:135–145. doi: 10.1016/s0091-3057(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Behavioral and Cognitive Neuroscience Reviews. 2004;3:143–158. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Penrod RD, Reichel CM. Nicotine does not produce state-dependent effects on learning in a Pavlovian appetitive goal-tracking task in rats. Behav Brain Res. 2007;177:134–141. doi: 10.1016/j.bbr.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Wilkinson JL, Palmatier MI, Siebert HL, Wiltgen SM. Characteristics of nicotine’s ability to serve as a negative feature in a Pavlovian appetitive conditioning task in rats. Psychopharmacology. 2006;184:470–481. doi: 10.1007/s00213-005-0079-3. [DOI] [PubMed] [Google Scholar]
- Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz HMB, editors. Operant-Pavlovian Interactions. Erlbaum; NJ: 1977. pp. 67–97. [Google Scholar]
- Bormann NM, Overton DA. The relative salience of morphine and contextual cues as conditioned stimuli. Psychopharmacology. 1996;123:164–171. doi: 10.1007/BF02246173. [DOI] [PubMed] [Google Scholar]
- Daly JW, Fredholm BB. Caffeine – an atypical drug of dependence. Drug Alcohol Depend. 1998;51:199–206. doi: 10.1016/s0376-8716(98)00077-5. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Aparicio J, Rescorla RA. Transfer between Pavlovian facilitators and instrumental discriminative stimuli. Anim Learn Behav. 1988;16:385–291. [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: Implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Farwell BJ, Ayres JJB. Stimulus-reinforcer and response-reinforcer relations in the control of conditioned appetitive headpoking (“goal-tracking”) in rats. Learning and Motivation. 1979;10:295–312. [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analysis. 1986;25:277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Greeley J, Lê DA, Poulos CX, Cappell H. Alcohol is an effective cue in the conditional control of tolerance to alcohol. Psychopharmacology. 1984;83:159–162. doi: 10.1007/BF00429726. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Evans SM, Heishman SJ, Preston KL, Sannerud CA, Wolf B, Woodson PP. Low-dose caffeine discrimination in humans. J Pharmacol Exp Ther. 1990;252:970–978. [PubMed] [Google Scholar]
- Griffiths RR, Woodson PP. Reinforcing effects of caffeine in humans. J Pharmacol Exp Ther. 1988;246:21–29. [PubMed] [Google Scholar]
- Holtzman SG. Discriminative stimulus properties of caffeine in the rat: noradrenergic mediation. J Pharmacol Exp Ther. 1986;239:706–713. [PubMed] [Google Scholar]
- Holtzman SG. Discriminative stimulus effects of caffeine: tolerance and cross-tolerance with methylphenidate. Life Sci. 1987;40:381–389. doi: 10.1016/0024-3205(87)90140-8. [DOI] [PubMed] [Google Scholar]
- Jaeger TV, Mucha RF. A taste aversion model of drug discrimination learning: training drug and condition influence rate of learning, sensitivity and drug specificity. Psychopharmacology. 1990;100:145–150. doi: 10.1007/BF02244397. [DOI] [PubMed] [Google Scholar]
- Maes JH, Vossen JM. Conditional control by midazolam and amphetamine in a rapid appetitive discrimination procedure. Eur J Pharmacol. 1997;319:5–11. doi: 10.1016/s0014-2999(96)00953-3. [DOI] [PubMed] [Google Scholar]
- Mariathasan EA, Stolerman IP. Drug discrimination studies in rats with caffeine and phenylpropanolamine administered separately and as mixtures. Psychopharmacology. 1992;109:99–106. doi: 10.1007/BF02245486. [DOI] [PubMed] [Google Scholar]
- Modrow HE, Holloway FA, Carney JM. Caffeine discrimination in the rat. Pharmacol Biochem Behav. 1981;14:683–688. doi: 10.1016/0091-3057(81)90131-3. [DOI] [PubMed] [Google Scholar]
- Mumford GK, Holtzman SG. Qualitative differences in the discriminative stimulus effects of low and high doses of caffeine in the rat. J Pharmacol Exp Ther. 1991;258:857–865. [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. Eur J Pharmacol. doi: 10.1016/j.ejphar.2007.01.046. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW. Caffeine drug discrimination in humans: Acquisition, specificity and correlation with self-reports. J Pharmacol Exp Ther. 1992;261:885–894. [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances reward-related learning in the rat. Neuropsychopharmacology. 2003;28:1264–1271. doi: 10.1038/sj.npp.1300173. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology. 2004;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Bevins RA. Facilitation by drug states does not depend on acquired excitatory strength. Behav Brain Res. 2007;176:292–301. doi: 10.1016/j.bbr.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Peterson JL, Wilkinson JL, Bevins RA. Nicotine serves as a feature-positive modulator of Pavlovian appetitive conditioning in rats. Behav Pharmacol. 2004;15:183–194. [PubMed] [Google Scholar]
- Palmatier MI, Wilkinson JL, Bevins RA. Stimulus properties of nicotine, amphetamine, and chlordiazepoxide as positive features in a Pavlovian appetitive discrimination task in rats. Neuropsychopharmacology. 2005;30:731–741. doi: 10.1038/sj.npp.1300629. [DOI] [PubMed] [Google Scholar]
- Parker BK, Schaal DW, Miller M. Drug discrimination using a Pavlovian conditional discrimination paradigm in pigeons. Pharmacol Biochem Behav. 1994;49:955–960. doi: 10.1016/0091-3057(94)90249-6. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Powell KR, Koppelman LF, Holtzman SG. Differential involvement of dopamine in mediating the discriminative stimulus effects of low and high doses of caffeine in rats. Behav Pharmacol. 1999;10:707–716. doi: 10.1097/00008877-199912000-00001. [DOI] [PubMed] [Google Scholar]
- Revusky S, Coombes S, Pohl RW. Drug states as discriminative stimuli in a flavor-aversion learning experiment. J Comp Physiol Psychol. 1982;96:200–211. doi: 10.1037/h0077870. [DOI] [PubMed] [Google Scholar]
- Simon NW, Setlow B. Post-training amphetamine administration enhances memory consolidation in appetitive Pavlovian conditioning: Implications for drug addiction. Neurobiol Learn Mem. 2006;86:305–310. doi: 10.1016/j.nlm.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Sokolowska M, Siegel S, Kim JA. Intraadministration associations: Conditional hyperalgesia elicited by morphine onset cues. J Exp Psychol Anim Behav Process. 2002;28:309–320. [PubMed] [Google Scholar]
- Troisi JR, Akins C. The discriminative stimulus effects of cocaine in a Pavlovian sexual approach paradigm in male Japanese quail. Exp Clin Psychopharmacol. 2004;12:237–242. doi: 10.1037/1064-1297.12.4.237. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Murray JE, Li C, Wiltgen SM, Penrod RD, Berg SA, Bevins RA. Interoceptive Pavlovian conditioning with nicotine as the conditional stimulus varies as function of number of conditioning trials and unpaired sucrose deliveries. Behav Pharmacol. 2006;17:161–172. doi: 10.1097/01.fbp.0000197456.63150.cd. [DOI] [PubMed] [Google Scholar]


