Abstract
γ-Secretase is involved in the production of amyloid β-peptide, which is the principal component of amyloid plaques in the brains of patients with Alzheimer disease. γ-Secretase is a complex composed of presenilin (PS), nicastrin, anterior pharynx-defective phenotype 1 (Aph1) and PS enhancer 2 (Pen2). We previously proposed a mechanism of complex assembly by which unassembled subunits are retained in the endoplasmic reticulum (ER) and only the fully assembled complex is exported from the ER. We have now identified Retention in endoplasmic reticulum 1 (Rer1) as a protein that is involved in the retention/retrieval of unassembled Pen2 to the ER. Direct binding of unassembled Pen2 to Rer1 is mediated by the first transmembrane domain of Pen2, and a conserved asparagine in this domain is required. Downregulation of Rer1 leads to increased surface localization of Pen2, whereas overexpression of Rer1 stabilizes unassembled Pen2. To our knowledge, Rer1 is the first identified interaction partner of mammalian transmembrane-based retention/retrieval signals.
Keywords: Alzheimer disease, γ-secretase, ER retention, ER retrieval, Pen2
Introduction
γ-Secretase is one of two proteases involved in the production of amyloid β-peptide, which is the principal component of amyloid plaques in the brains of patients with Alzheimer disease. γ-Secretase is a high-molecular-weight complex composed of presenilin (PS) 1 or PS2, nicastrin (Nct), anterior pharynx-defective phenotype 1 (Aph1) and PS enhancer 2 (Pen2; for a review, see Haass, 2004).
γ-Secretase assembles in the endoplasmic reticulum (ER; Kim et al, 2004; Capell et al, 2005), but how the complex is assembled and transported to the plasma membrane is not fully understood. We recently proposed a model for γ-secretase complex assembly and transport (Kaether et al, 2004, 2006). According to this model, the assembly of γ-secretase is controlled by mechanisms similar to those that mediate the assembly of ion channels and multimeric cell-surface receptors. Here, control mechanisms ensure that only fully assembled complexes leave the ER and travel to the plasma membrane. Retention—we use the term retention for retention, retrieval or a combination of both—of unassembled subunits is achieved by exposing ER-retention signals that can be cytosolic (Zerangue et al, 1999) or within transmembrane domains (TMDs; Bonifacino et al, 1991). After complex assembly, these retention signals are masked and ER export is permitted. As predicted from this model, a new type of ER-retention signal was identified in the carboxyl terminus of PS1 (Kaether et al, 2004). However, at present, the cellular mechanism by which unassembled γ-secretase complex components are retained in or retrieved to the ER is not known. Retention in endoplasmic reticulum 1 (Rer1p) is a 22-kDa yeast protein involved in the retrieval of several ER-localized proteins and in the retrieval of unassembled subunits of multimeric complexes (Sato et al, 2003). Its mammalian homologue might be a candidate for ER retention/retrieval of unincorporated γ-secretase complex components. Rer1p is predicted to have a W-shaped topology with four TMDs, and amino and C termini located in the cytosol (Boehm et al, 1994), and recognizes polar amino acids in TMDs (Sato et al, 2003). Such TMD-based retention signals have also been described for several subunits of mammalian multimeric complexes (Bonifacino et al, 1991; Reth et al, 1991; Hennecke & Cosson, 1993), but no interaction partners or molecular machinery have been described. A human homologue of Rer1p was shown to rescue a yeast strain defective in Rer1p (Füllekrug et al, 1997), but its function in mammalian cells has not been described so far.
We have now investigated ER retention of Pen2 and shown that human Rer1 is involved in this process by binding to a signal in the first TMD (TM1) of Pen2.
Results And Discussion
The TM1 of Pen2 contains an ER-retention motif
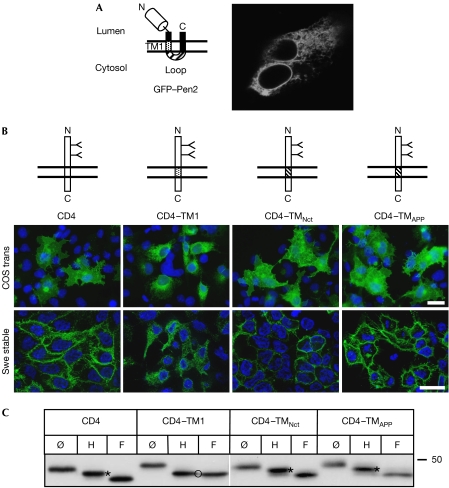
First, we confirmed that unassembled Pen2 localized to the ER. For this, we transiently overexpressed a green fluorescent protein (GFP)–Pen2 fusion protein (Fig 1A) in human embryonic kidney 293 cells (HEK293). This showed prominent ER staining, consistent with previous findings (Fig 1A; Bergman et al, 2004; Crystal et al, 2004). Then, the domains in Pen2 that are responsible for its ER retention were identified by using cluster of differentiation 4 (CD4), a type I reporter protein previously used to identify ER-retention signals (Zerangue et al, 1999; Kaether et al, 2004). Consistent with previous results (Zerangue et al, 1999; Kaether et al, 2004), transient expression of CD4 in COS cells or stable expression in HEK293 Swe cells led to the accumulation of the protein in the plasma membrane (Fig 1B). Addition of the Pen2 intracellular loop to the C-terminus of CD4 had no effect on localization, as the protein was efficiently transported to the plasma membrane (data not shown). Swapping the second TMD of Pen2 in tumour necrosis factor (TNF)α, a type II reporter, did not prevent the protein from leaving the ER (supplementary Fig 1 online). By contrast, when the CD4 TMD was replaced by the TM1 of Pen2, the resulting construct CD4–TM1 showed prominent ER staining in transiently transfected COS and stably transfected Swe cells, suggesting that TM1 mediates ER retention (Fig 1B). ER localization of CD4–TM1 was not due to misfolding, destruction of an ER-export motif or other motif important for cell surface localization of CD4, as insertion of the TMD of Nct or amyloid precursor protein (APP) did not lead to ER retention of CD4 (Fig 1B, for CD4–TMNct; see also Capell et al, 2005). Further evidence of ER retention of CD4–TM1 was obtained by deglycosylation experiments (Fig 1C). Similarly to CD4, CD4–TMNct and CD4–TMAPP were endoglycosidase H (endoH) resistant, indicating transport to the late Golgi and beyond. By contrast, CD4–TM1 remained endoH sensitive, confirming that it did not reach late Golgi compartments (Fig 1C). Fluorescence-activated cell sorting (FACS) of HEK cells stably expressing CD4 variants was used as an additional method for demonstrating selective retention of CD4–TM1. Cells were labelled with CD4 antibodies with or without permeabilization, sorted with FACS and then the fraction of plasma membrane-localized antigen against total CD4 antigen was determined. In the case of CD4, CD4–TMNct and CD4–TMAPP, 71±2%, 67±3% and 73% of total CD4 antigen were at the plasma membrane, respectively, showing efficient plasma membrane transport. By contrast, CD4–TM1-expressing cells had only 14±1% of total CD4 antigen at the plasma membrane, showing efficient intracellular retention and fully corroborating the results from the immunofluorescence and deglycosylation experiments. Together, these data show that the TM1 of Pen2 is sufficient to mediate ER retention of a reporter protein.
Figure 1.
The first transmembrane domain of Pen2 contained an endoplasmic reticulum-retention signal. (A) GFP–Pen2 topology (left) and confocal image of living cells transiently expressing GFP–Pen2 (right). (B) CD4 constructs were used to determine ER-retention signals. The two N-glycosylation sites in CD4 are indicated. Transiently transfected COS cells or Swe cells stably expressing the indicated constructs were processed for immunofluorescence with CD4 antibodies. Images were exposed and processed identically. For Swe cells, single confocal-like apotome sections are shown, resulting in a ring-like plasma membrane-staining. (C) Deglycosylation experiments confirmed ER retention of the CD4–TM1 construct. Lysates of Swe cells stably expressing CD4 variants, as indicated, were immunoprecipitated with CD4 antibodies (circle with a slash), or immunoprecipitated and digested with endoH (H) or N-glycosidase F (F). Asterisks, EndoH resistant, indicating transport through the Golgi; circle, EndoH sensitive, indicating ER retention. The white line indicates assembly from different areas of the blot. APP, amyloid precursor protein; CD4, cluster of differentiation 4; endoH, endoglycosidase H; ER, endoplasmic reticulum; GFP, green fluorescent protein; Nct, nicastrin; Pen2, presenilin enhancer 2; TM1, first TMD of Pen2; TMD, transmembrane domain.
The distal part of TM1 mediates ER retention
To specify further the domain important for the retention of CD4–TM1, we replaced parts of the CD4 TMD with corresponding parts of the Pen2 TM1 (schematized in supplementary Fig 1 online). CD4 variants were transfected in COS cells and the subcellular distribution of the fusion proteins was analysed by immunofluorescence. Only the C-terminal part of TM1 retained the corresponding CD4 construct in the ER (supplementary Fig 1 online).
An asparagine in TM1 is part of an ER-retention signal
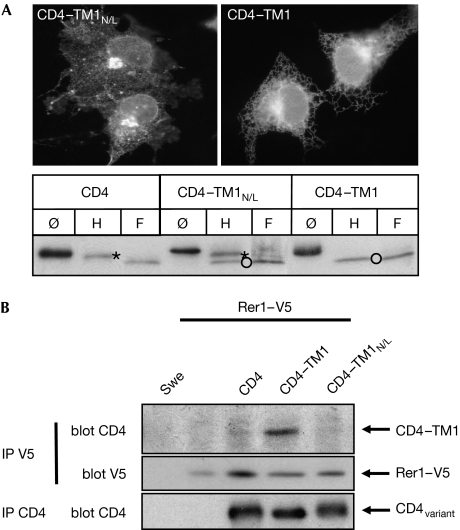
Charged or polar amino acids in TMDs have been shown to be crucially involved in ER retention (Bonifacino et al, 1991; Sato et al, 2003). The TM1 of Pen2 contains an asparagine (N) in the domain responsible for ER retention, which is highly conserved across species. To test the involvement of this polar amino acid in ER retention, we mutated it to leucine (L) in CD4–TM1 and analysed the subcellular distribution of the mutant by immunofluorescence and deglycosylation experiments. Mutation of N to L partly destroyed the ER-retention motif, as shown by immunofluorescence (Fig 2A). This was confirmed by deglycosylation experiments showing equal amounts of endoH-resistant and nonresistant CD4–TM1N/L, in contrast to the fully endoH-sensitive CD4–TM1 (Fig 2A). This indicates that the conserved N residue is important but not fully sufficient for ER retention.
Figure 2.
Rer1 retained CD4–TM1 in the endoplasmic reticulum. (A) Retention of CD4–TM1 was dependent on a crucial asparagine in TM1. COS cells were transiently transfected with CD4–TM1N/L or CD4–TM1 and processed for CD4 immunofluorescence. Deglycosylation experiments verified partial destruction of the ER-retention motif in CD4–TM1N/L. Cell lysates of Swe cells stably expressing the indicated constructs were treated as in Fig 1C. Asterisks, endoH resistant; circles, endoH sensitive. (B) Rer1 bound to CD4–TM1 is dependent on the asparagine within TM1. CHAPSO cell lysates from Swe cells and Swe cells stably expressing Rer1–V5 alone or together with CD4 variants were immunoprecipitated with a V5 antibody and blotted as indicated. Expression of CD4 variants was analysed by IP, followed by western blot using CD4 antibodies. CD4, cluster of differentiation 4; endoH, endoglycosidase H; F, N-glycosidase F; IP, immunoprecipitation; L, leucine; N, asparagine; Pen2, presenilin enhancer 2; TM1, first TMD of Pen2; TMD, transmembrane domain.
Retention of CD4–TM1 is mediated by Rer1
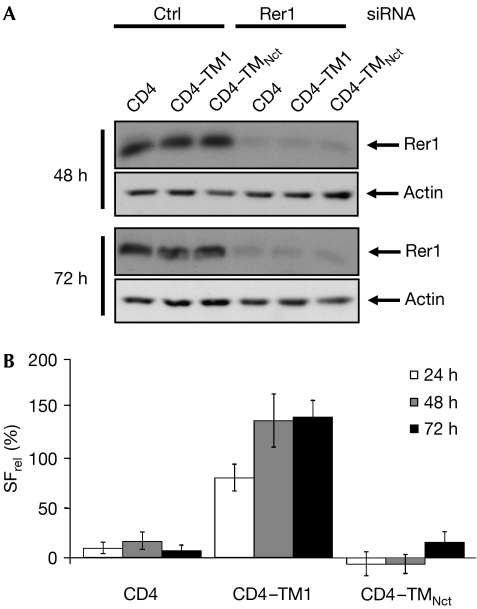
The yeast protein Rer1p was shown to mediate ER retention of proteins by binding to polar amino acids within TMDs (Sato et al, 2003). To test whether its human homologue Rer1 is involved in the retention of CD4–TM1, we stably expressed V5-tagged human RER1 together with CD4 variants and carried out co-immunoprecipitation experiments (Fig 2B). CD4–TM1 co-precipitated with Rer1, whereas CD4 alone or CD4–TM1N/L did not co-precipitate with Rer1. This suggests that Rer1 binds to CD4–TM1 and that this is dependent on the crucial asparagine in TM1. To test directly whether endogenous Rer1 is involved in ER retention of CD4–TM1, we used short interfering RNA (siRNA) and FACS analysis to investigate the plasma membrane localization of CD4 variants. Cells stably expressing CD4, CD4–TM1 or CD4–TMNct were transfected with Rer1 or control siRNA and collected at different time points. We verified that Rer1 was efficiently downregulated on Rer1 siRNA treatment, but not by using control siRNA (Fig 3A). CD4 surface labelling was determined with FACS, and the relative changes in surface labelling between Rer1 and control transfected cells were determined at each time point for each cell line (Fig 3B). Downregulation of Rer1 did not affect the secretory pathway, as transport of CD4 and CD4–TMNct to the plasma membrane was not significantly affected (Fig 3B). By contrast, in cells transfected with Rer1 siRNA, an 80% increase in CD4–TM1 plasma membrane staining was observed after 24 h. This increased to 137% after 48 h and 141% after 72 h (Fig 3B). These data strongly indicate that Rer1 is involved in ER retention of CD4–TM1.
Figure 3.
Endogenous Rer1 was involved in the retention of CD4–TM1. (A) Cells stably expressing the indicated CD4 variant were transfected with siRNA, lysed after the indicated time points and blotted for Rer1 and actin as a loading control. Rer1 is significantly downregulated in Rer1 but not control (Ctrl) siRNA-transfected cells. (B) Cells from (A) were analysed by fluorescence-activated cell sorting after the indicated time points using CD4 antibodies. The ratio of surface labelling of control and Rer1 siRNA-transfected cells (SFrel) is shown, where 0% indicates no change in surface staining between Rer1 and control siRNA-transfected cells. Note that absolute surface levels between CD4– and CD4–TM1-expressing cells differ strongly, as 86% of CD4–TM1 is retained intracellularly, whereas 71% of CD4 is at the plasma membrane. CD4, cluster of differentiation 4; Nct, nicastrin; Pen2, presenilin enhancer 2; siRNA, short interfering RNA; TM1, first TMD of Pen2; TMD, transmembrane domain.
Rer1 selectively binds to unassembled Pen2
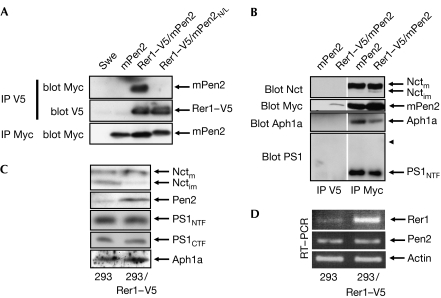
Next, we wanted to test whether Rer1 would also bind to Pen2 itself. Rer1–V5 was stably expressed together with the N-terminally Myc-tagged Pen2 or Pen2N/L. Both variants assembled into a functional γ-secretase complex, indicating correct folding and function (supplementary Fig 2D online and data not shown). Co-immunoprecipitation experiments indicated that Rer1 bound to Pen2 dependent on the crucial asparagine in TM1 (Fig 4A), validating the data obtained with the CD4 reporter protein. To analyse whether Rer1 binds to γ-secretase complex-assembled or unassembled Pen2, co-immunoprecipitations were carried out in cells stably expressing Rer1–V5 and Myc–Pen2 variants (Fig 4B). Immunoprecipitation with a V5 antibody precipitated Pen2, but not Nct, Aph1a or PS1, the other γ-secretase complex components, indicating that Rer1V5 binds to only unassembled Pen2 (Fig 4B). By contrast, immunoprecipitation with a Myc antibody co-precipitated Myc–Pen2 together with mature Nct, Aph1 and PS1 (Fig 4B). These data showed that there was a fraction of Myc–Pen2 in the cell assembled into a γ-secretase complex and not binding to Rer1, and another fraction that was unassembled and bound Rer1. Unassembled Pen2N/L, which is not able to bind to Rer1 (Fig 4A), is expected to escape the ER-retention mechanism and to accumulate at the plasma membrane. Indeed, stably expressed GFP-tagged Pen2 accumulated 1.6 times more at the plasma membrane when the crucial asparagine in TM1 was mutated, supporting the data obtained with the CD4 variants (supplementary Fig 2A,C online).
Figure 4.
Rer1 selectively bound to unassembled Pen2 and retained it in the endoplasmic reticulum. (A) Rer1 immunoprecipitated Pen2 dependent on the asparagine within TM1. Lysates from Swe cells and Swe cells stably expressing the indicated proteins were immunoprecipitated with the V5 antibody and blotted as indicated. Expression of mPen2 variants was analysed by IP/blot using Myc antibodies. Note that Pen2N/L has a slower mobility compared with Pen2. (B) Rer1 bound to unassembled Pen2. Lysates from Swe cells stably expressing Myc-tagged Pen2 (mPen2) alone or together with Rer1–V5 were immunoprecipitated with a V5 antibody and subsequently with Myc antibodies and blotted for Nct, PS1, Aph1a and Myc. The arrowhead indicates the position of the PS1 holoprotein, not present in both co-immunoprecipitations. The white line indicates the assembly from different areas of the blot. (C) Rer1–V5 stabilizes endogenous Pen2. Membrane preparations of HEK293 cells and HEK293 cells overexpressing Rer1–V5 were probed for Nct, Pen2, PS1NTF, PS1CTF and Aph1a. (D) RT–PCR of cells with or without stably expressing Rer–V5 was carried out for actin, Rer1 and Pen2 messenger RNA. Aph, anterior pharynx-defective phenotype; CTF, C-terminal fragment; HEK293, human embryonic kidney 293 cells; IP, immunoprecipitation; subscript im, immature; L, leucine; subscript m, mature; mPen2, Myc–Pen2; N, asparagine; Nct, nicastrin; NTF, N-terminal fragment; Pen2, PS enhancer 2; PS, presenilin; TM1, first TMD of Pen2; TMD, transmembrane domain.
In cells stably overexpressing Rer1, endogenous levels of Pen2 were increased (Fig 4C), whereas levels of Pen2 messenger RNA were unchanged (Fig 4D), indicating stabilization of unassembled Pen2 by binding to Rer1 and confirming an interaction of the two proteins. The levels of mature Nct, PS1 fragments and Aph1a, indicative of the total amount of mature γ-secretase complex, were unchanged, further supporting the data that Rer1 selectively interacts with unassembled Pen2 (Fig 4C). We noted that, on overexpression of Rer1, the immature/mature Nct ratio consistently changed towards mature Nct, the complex assembled form (Fig 4C). This might be a consequence of the increased Pen2 levels, which might increase γ-secretase assembly and export out of the ER. Indeed, Pen2 was shown to be rate limiting for γ-secretase complex assembly (Kimberly et al, 2003; Takasugi et al, 2003).
Together, these data indicate that unassembled Pen2 is retained/retrieved in the ER by Rer1, a process that requires a crucial asparagine in the TM1 of Pen2. Rer1 specifically binds to and retains unassembled Pen2, supporting our hypothesis that a quality control system ensures that only fully assembled γ-secretase complex—but not unassembled subunits—can leave the ER. Furthermore, we identify Pen2 as the first substrate of mammalian Rer1, opening the possibility to study previously uncharacterized ER-retention mechanisms governed by TM-based retention signals.
Methods
Antibodies and cell lines. Nct, Aph1a, PS1 N- and C-terminal fragment, GFP and CD4 were detected as described previously (Kaether et al, 2004). For FACS sorting, CD4–APC (Miltenyi Biotec, Bergisch Gladbach, Germany) was used. For the detection of Myc-tagged Pen2 and haemagglutinin (HA)-tagged TNFα, monoclonal 9E10 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-HA (Sigma) were used, respectively. Rer1 was detected using a custom-made affinity-purified polyclonal antiserum against the C terminus of human Rer1 (Eurogentec, Seraing, Belgium). HEK293 cells stably expressing Swedish mutant APP (Swe) were described previously (Citron et al, 1992).
Complementary DNA constructs and transfections. Human RER1 was amplified from a brain cDNA library, by using standard PCR and appropriate primers that allowed subcloning into pcDNA6/V5-His (Invitrogen, Karlsruhe, Germany). TNFα–HA was obtained from S. Lichtenthaler and CD4–TMNct from A. Capell (Capell et al, 2005). For siRNA approaches, a mixture of four siRNA duplexes against Rer1 and control siRNA (both from Dharmacon, Lafayette, CO, USA) were transfected by using Amaxa nucleofector technology. CD4 and TNFα fusion constructs were cloned by using standard molecular cloning techniques and pcDNA3.1/Hygro (Invitrogen). Amino-acid sequences of TMDs and adjacent domains are shown in supplementary Fig 1A online. Mutations were introduced using the QuikChange site-directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA). N-terminally tagged Myc–Pen2 and GFP–Pen2 were cloned using PCR and pCMV-Myc and pEGFP-C1, respectively (both Clontech, Mountain View, CA, USA). Cells were transfected with Lipofectamine 2000 (Invitrogen). Pen2 variants were stably expressed in HEK293 cells stably expressing Pen2 siRNA (Prokop et al, 2004). Primer sequences and cloning details are available on request.
Co-immunoprecipitation. Co-immunoprecipitation was carried out from cell lysates extracted in 2% CHAPSO/citrate (pH 6.4) and protease inhibitor mix with antibodies as indicated. Immunoprecipitated proteins were separated on 8% or 10% SDS–polyacrylamide gel electrophoresis gels, 11% urea gels or 10–20% Tris-tricine gels (Invitrogen), and then transferred to polyvinylidene difluoride membranes. Membranes were cut at appropriate positions and blotted with antibodies as indicated.
Deglycosylation and cell surface biotinylation. Deglycosylation and cell surface biotinylation experiments were carried out as described previously (Kaether et al, 2002). Total GFP–Pen2 (from direct lysate) and cell surface levels (from streptavidin precipitation) on western blots were quantified using a Fluorchem 4800 system (Alpha Innotech, San Leandro, CA, USA).
Fluorescence-activated cell sorting analysis. FACS analysis was carried out by using a BD FACSCalibur and standard protocols. The fractions of CD4 antigen at the plasma membrane against total were determined using a Cytofix/Cytoperm kit from BD (Heidelberg, Germany) in three independent experiments. The relative increase in surface fluorescence after siRNA transfection was measured as follows. The amount of CD4 antigen at the plasma membrane was determined using FACS after control siRNA transfection (CD4ctrl) and Rer1 siRNA transfection (CD4Rer1). The relative increase was then calculated by CD4Rer1/CD4ctrl-1.
Microscopy. Immunofluorescence was carried out using standard protocols and Alexa 555- or 586-labelled secondary antibodies (Molecular Probes, Breda, The Netherlands). Microscopy and image acquisition was carried out as described previously (Kaether et al, 2004), with the modification that for some images a Zeiss Apotome was used (Carl Zeiss, Jena, Germany).
Reverse transcription–PCR. Total RNA was isolated using NucleoSpin® RNAII (Macherey-Nagel, Düren, Germany). RT–PCR was carried out using the ThermoScript™ RT–PCR system (Invitrogen) and primers specific for human Rer1, Pen2 and β-actin.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/v8/n8/extref/7401027-s1.pdf).
Note added in proof. During the revision of this paper, a study suggesting a binding of Rer1 to Nct, but not to Pen2, was presented, a finding not supported by our data (Spasic et al, 2007).
Supplementary Material
supplementary Information
Acknowledgments
We thank the Hans & Ilse Breuer Foundation for the confocal microscope, A. Capell for CD4–TMNct cDNA, S. Lichtenthaler for TNFα cDNA and S. Lammich for the brain cDNA library. This work was supported by the Molecular Medicine Program of the Medical Faculty (FöFoLe) of the Ludwigs-Maximilians-Universität München (to J.S.) and grants from the Deutsche Forschungsgemeinschaft (CIPSM to C.H., SFB596 to C.H. and H.S., and SFB604 to C.K.).
References
- Bergman A, Hansson EM, Pursglove SE, Farmery MR, Lannfelt L, Lendahl U, Lundkvist J, Naslund J (2004) Pen-2 is sequestered in the endoplasmic reticulum and subjected to ubiquitylation and proteasome-mediated degradation in the absence of presenilin. J Biol Chem 279: 16744–16753 [DOI] [PubMed] [Google Scholar]
- Boehm J, Ulrich HD, Ossig R, Schmitt HD (1994) Kex2-dependent invertase secretion as a tool to study the targeting of transmembrane proteins which are involved in ER → Golgi transport in yeast. EMBO J 13: 3696–3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Cosson P, Shah N, Klausner RD (1991) Role of potentially charged transmembrane residues in targeting proteins for retention and degradation within the endoplasmic reticulum. EMBO J 10: 2783–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capell A, Beher D, Prokop S, Steiner H, Kaether C, Shearman MS, Haass C (2005) γ-Secretase complex assembly within the early secretory pathway. J Biol Chem 280: 6471–6478 [DOI] [PubMed] [Google Scholar]
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ (1992) Mutation of the β-amyloid precursor protein in familial Alzheimer's disease increases β-protein production. Nature 360: 672–674 [DOI] [PubMed] [Google Scholar]
- Crystal AS, Morais VA, Fortna RR, Carlin D, Pierson TC, Wilson CA, Lee VM, Doms RW (2004) Presenilin modulates Pen-2 levels posttranslationally by protecting it from proteasomal degradation. Biochemistry 43: 3555–3563 [DOI] [PubMed] [Google Scholar]
- Füllekrug J, Boehm J, Rottger S, Nilsson T, Mieskes G, Schmitt HD (1997) Human Rer1 is localized to the Golgi apparatus and complements the deletion of the homologous Rer1 protein of Saccharomyces cerevisiae. Eur J Cell Biol 74: 31–40 [PubMed] [Google Scholar]
- Haass C (2004) Take five-BACE and the γ-secretase quartet conduct Alzheimer's amyloid β-peptide generation. EMBO J 23: 483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke S, Cosson P (1993) Role of transmembrane domains in assembly and intracellular transport of the CD8 molecule. J Biol Chem 268: 26607–26612 [PubMed] [Google Scholar]
- Kaether C, Lammich S, Edbauer D, Ertl M, Rietdorf J, Capell A, Steiner H, Haass C (2002) Presenilin-1 affects trafficking and processing of βAPP and is targeted in a complex with nicastrin to the plasma membrane. J Cell Biol 158: 551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaether C, Capell A, Edbauer D, Winkler E, Novak B, Steiner H, Haass C (2004) The presenilin C-terminus is required for ER-retention, nicastrin-binding and γ-secretase activity. EMBO J 23: 4738–4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaether C, Haass C, Steiner H (2006) Assembly, trafficking and function of γ-secretase. Neurodegener Dis 3: 275–283 [DOI] [PubMed] [Google Scholar]
- Kim SH, Yin YI, Li YM, Sisodia SS (2004) Evidence that assembly of an active γ-secretase complex occurs in the early compartments of the secretory pathway. J Biol Chem 279: 48615–48619 [DOI] [PubMed] [Google Scholar]
- Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ (2003) γ-Secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc Natl Acad Sci USA 100: 6382–6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop S, Shirotani K, Edbauer D, Haass C, Steiner H (2004) Requirement of PEN-2 for stabilization of the presenilin N-/C-terminal fragment heterodimer within the γ-secretase complex. J Biol Chem 279: 23255–23261 [DOI] [PubMed] [Google Scholar]
- Reth M, Hombach J, Wienands J, Campbell KS, Chien N, Justement LB, Cambier JC (1991) The B-cell antigen receptor complex. Immunol Today 12: 196–201 [DOI] [PubMed] [Google Scholar]
- Sato K, Sato M, Nakano A (2003) Rer1p, a retrieval receptor for ER membrane proteins, recognizes transmembrane domains in multiple modes. Mol Biol Cell 14: 3605–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasic D, Raemaekers T, Dillen K, Declerck I, Baert V, Serneels L, Fullekrug J, Annaert W (2007) Rer1p competes with APH-1 for binding to nicastrin and regulates γ-secretase complex assembly in the early secretory pathway. J Cell Biol 176: 629–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T (2003) The role of presenilin cofactors in the γ-secretase complex. Nature 422: 438–441 [DOI] [PubMed] [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY (1999) A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron 22: 537–548 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Information