Abstract
The stable subdivision of Drosophila limbs into anterior and posterior compartments is a consequence of asymmetrical signalling by Hedgehog (Hh), from the posterior to anterior cells. The activity of the homeodomain protein Engrailed in posterior cells helps to generate this asymmetry by inducing the expression of Hh in the posterior compartment and, at the same time, repressing the expression of the essential downstream component Cubitus interruptus (Ci). Therefore, only anterior cells that receive the Hh signal across the compartment boundary will respond by stabilizing Ci. Here, we describe a new molecular mechanism that helps to maintain the Hh-expressing and Hh-responding cells in different non-overlapping cell populations. Master of thickveins (mtv)—a target of Hh activity encoding a nuclear zinc-finger protein—is required to repress hh expression in anterior cells. Mtv exerts this action in a protein complex with Groucho (Gro)—the founding member of a superfamily of transcriptional corepressors that are conserved throughout eukaryotes. Therefore, Hh restricts its own expression domain in the Drosophila wing through the activity of Mtv and Gro.
Introduction
The limb primordia of Drosophila are subdivided into adjacent territories called compartments—that is, cell populations that do not mix during development (García-Bellido et al, 1973). Anterior and posterior cells in limb primordia acquire specific fates during embryogenesis through the activity of the homeodomain proteins Engrailed (En) and Invected in posterior cells (Tabata et al, 1995). Soon after the discovery that anterior and posterior cells are separated by a lineage restriction boundary (García-Bellido et al, 1973), it was proposed that this boundary acts as an organizing centre in the developing appendages (Crick & Lawrence, 1975). Molecular analyses have shown that specialized cells are established along the anterior–posterior boundary as a consequence of asymmetrical signalling by the diffusible protein Hedgehog (Hh), from the posterior to anterior cells (Basler & Struhl, 1994; Tabata & Kornberg, 1994). En induces expression of Hh in the posterior compartment and represses the expression of the essential downstream component of the Hh signalling pathway Cubitus interruptus (Ci). Ci can exist in two forms: a repressor form (Cirep) is generated in cells that do not receive the Hh signal; and an activator form of Ci (Ciact) is generated in cells that receive the Hh signal (Fig 1I; Aza-Blanc et al, 1997; Ohlmeyer & Kalderon, 1998). Both forms of Ci control the expression of the secreted signalling molecule Decapentaplegic (Dpp) in a thin stripe of anterior cells along the anterior–posterior boundary (Méthot & Basler, 1999). Dpp acts as a symmetrical long-range morphogen. By this mechanism the originally asymmetrical subdivision of the limb primordium eventually leads to the establishment of a symmetrical organizing gradient of Dpp.
Figure 1.
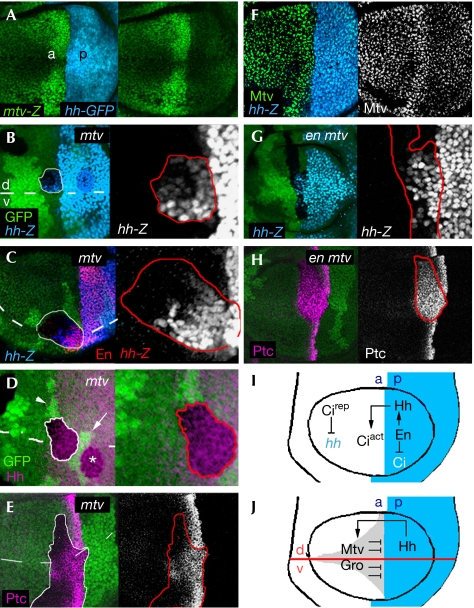
Ectopic expression of hh in the absence of mtv activity. (A) Wing disc labelled to visualize expression of the mtv-lacZ reporter gene (antibody to β-Gal, green) in an hh-GAL4;UAS-GFP background (GFP, blue). (F) Wing disc labelled to visualize expression of a hh-lacZ reporter gene (antibody to β-gal, blue) and Mtv protein expression (green or white). (B–E,G,H) Clones of cells mutant for mtv (mtv6; B–E) or for both engrailed/invected and mtv (Df(2R)en[E] mtv6; G,H) and labelled by the absence of the GFP marker (green). The contour of the clone is marked by a red or white line. hh-lacZ (blue or white; B,C,G), Engrailed (En, red; C), Hedgehog (Hh, purple; D) or Patched (Ptc, purple or white; E,H) is ectopically expressed in a clone abutting the anterior–posterior (ap) and dorsal–ventral (dv; dashed lines) boundaries. Note in (D) that the marked clone with ectopic Hh protein expression was born in the anterior (a) compartment (twin clone is marked by an arrowhead) but crosses into the posterior (p) compartment. A neighbouring p clone (white asterisk) and its twin (white arrow) are shown. Violation of the ap compartment was very frequently observed in these clones (data not shown). (I,J) Two different mechanisms to repress hh expression in a cells. (I) En expression in p cells induces hh expression and represses cubitus interruptus (ci) expression. Hh activity in a cells stabilizes the transcriptional activator form of Ci (Ciact). In the absence of Hh signal, Ci is transformed to a transcriptional repressor (Cirep). Cirep represses Hh expression. (J) Proposed model illustrating the role of Mtv and Gro in the repression of hh in a cells. GFP, green fluorescent protein; Gro, Groucho; Mtv, master of thickveins.
Asymmetrical signalling of Hh also relies on restricting the Hh-expressing cells to the posterior compartment (Fig 1I,J). Cirep has been shown to repress the expression of hh in anterior cells, mainly in those cells that do not receive the Hh signal (Fig 1I; Méthot & Basler, 1999). The transcriptional corepressor Groucho (Gro)—the founding member of a superfamily of corepressors that are conserved throughout eukaryotes—is also required to repress hh expression in anterior cells, but in cells that are close to the anterior–posterior boundary (Fig 1J; de Celis & Ruiz-Gomez, 1995; Apidianakis et al, 2001). Gro is recruited by several transcription factors to mediate various long-range repression mechanisms during embryonic and larval development of Drosophila. Here, we show that Master of thickveins (Mtv), a target of Hh activity encoding a nuclear zinc-finger protein (Funakoshi et al, 2001), and Gro take part in the same protein complex and mediate transcriptional repression of hh. Therefore, two different mechanisms are used in the Drosophila wing to restrict hh expression to the posterior compartment. Several signalling molecules have been shown to restrict their own expression or activity domains (Freeman & Gurdon, 2002); to our knowledge, this is the first time that Hh has been shown to be involved in restricting its own expression domain.
Results And Discussion
The anterior–posterior asymmetrical expression of mtv in the developing wing primordium (Fig 1A,F) follows that of Hh activity, and is a consequence of Hh increasing mtv expression at the anterior–posterior boundary and En reducing mtv expression in posterior cells (Funakoshi et al, 2001). To assess the role of mtv in wing development, clones of cells mutant for a loss-of-function allele of mtv—mtv6—were generated using the FLP/FRT technique. Clones were marked by the absence of the green fluorescent protein (GFP) marker. These mutant cells caused ectopic expression of hh in anterior cells (Fig 1B–D). Only those cells abutting or close to the anterior–posterior boundary were able to induce hh. We monitored the expression of the Hh target genes en and patched (ptc) in these clones. Again, loss of mtv caused an expansion of en and ptc expression domains in cells located in the same region (Fig 1C,E). Posterior clones did not have any effect on the expression of Hh target genes (data not shown).
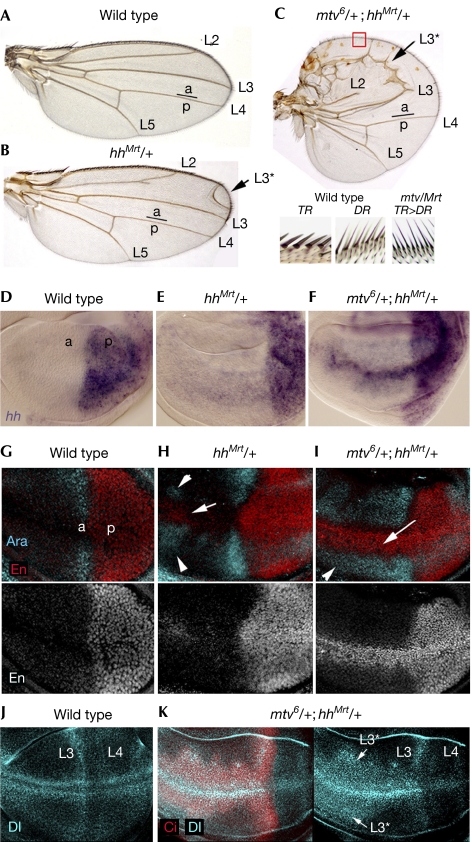
In the absence of mtv activity, hh is expressed in anterior cells. The hh gain-of-funcion allele Moonrat—hhMrt—also resulted in derepression of hh in anterior cells and caused duplication of anterior structures—that is, longitudinal veins (arrow in Fig 2B)—and enlargement of the anterior compartment (de Celis & Ruiz-Gomez, 1995; Felsenfeld & Kennison, 1995). We then investigated whether reduced levels of mtv activity would enhance the hhMrt phenotype. Loss of one copy of mtv strongly enhanced the hhMrt heterozygous adult wing phenotype (compare Fig 2B and C). In hhMrt heterozygous wing discs, slight ectopic expression of hh messenger RNA, En and Araucan (Ara), another target of Hh (Gomez-Skarmeta & Modolell, 1996), was observed in anterior cells (compare Fig 2D,G with E,H). Loss of one copy of mtv in this hhMrt heterozygous background increased the number of cells ectopically expressing hh mRNA, En and Ara and the levels of expression (Fig 2F,I). The ectopic expression of En (arrow in Fig 2I), a target of Hh required to specify the fate of wing margin bristles at the anterior–posterior boundary (Hidalgo, 1994), is consistent with the observation that bristles along the anterior wing margin (triple row bristles, TR) were transformed into more posteriorly located sensory organs (double row bristles, DR; Fig 2C). The enlargement of the expression domain of Ara (arrowheads in Fig 2I), a target of Hh that defines the position of the third longitudinal vein (L3; Fig 2A; Gomez-Skarmeta & Modolell, 1996), explains the ectopic L3 observed in mtv6hhMrt double heterozygous wings (arrow in Fig 2C). Delta protein is expressed along the presumptive longitudinal veins L3 and L4 in the wing primordium (Fig 2J). In mtv6hhMrt double heterozygous wing discs, Delta is ectopically expressed in the anterior compartment (Fig 2K), presumably in those cells that will give rise to the ectopic vein L3 observed in the resulting adult wings. Note the relatively normal levels of Delta protein expression in the posterior compartment of these discs.
Figure 2.
Genetic interactions between mtv and the hh gain-of-function allele Moonrat. (A–C) Cuticle preparations of wild type (A), hhMrt/+ (B) and mtv6/+; hhMrt/+ (C) adult wings. The adult wing is decorated by four longitudinal veins (L2–L5) and sensory organs along the margin. Note in (B) and (C) the ectopic vein L3 (L3*, marked by an arrow). In (C) the anterior compartment is enlarged. As shown in the magnification of the anterior wing margin (red box) at the bottom of the panel, the sensory organs located in the most anterior wing margin (Triple Row Bristles, TR) are transformed into more posteriorly located bristles (Double Row Bristles, DR). Anterior (a) and posterior (p) compartments are indicated. (D–K) Wild-type (D,G,J), hhMrt/+ (E,H) and mtv6/+; hhMrt/+ (F,I,K) wing discs labelled to visualize expression of hh messenger RNA (purple; D–F), Araucan (Ara, blue; G–I), Engrailed (red in the top panels, white in the bottom panels; G–I), Delta (Dl, blue; J,K) and Ci (red; K). Note ectopic expression of Dl in the anterior compartment, presumably marking the presumptive ectopic vein L3 (L3*, marked by an arrow) visualized in adult flies (C). Hh, Hedgehog; Mtv, master of thickveins.
hh is a target of En in posterior cells; therefore, ectopic expression of hh might be a consequence of derepression of En in mtv mutant clones. However, clones lacking mtv and en activities still showed the ability to cause ectopic expression of hh and its target gene ptc (Fig 1G,H) in the anterior compartment. Anterior clones lacking en activity alone did not have any effect on the expression of hh (supplementary Fig 1 online). Together, these results indicate that Mtv is required to repress hh expression in anterior cells, independently of En.
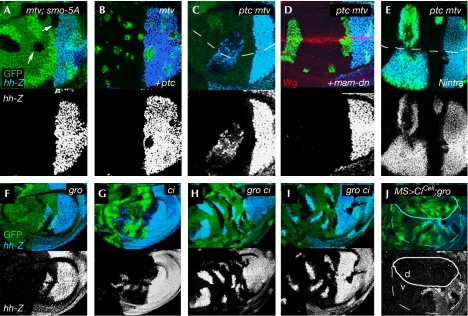
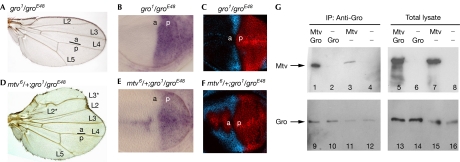
Gro activity has previously been shown to be required for the repression of hh transcription in anterior cells (de Celis & Ruiz-Gomez, 1995; Apidianakis et al, 2001). Gro is ubiquitously expressed in the wing disc, but it is required only at the anterior–posterior boundary. This topological requirement seems to be similar to that of Mtv (compare Fig 1B–D with Fig 4F; note that clones were marked in this case by the presence of two copies of the GFP marker), thus indicating that Gro and Mtv might work together to repress hh. Interestingly, gro and mtv genetically interact in vivo. Transheterozygous gro1/groE48 adult wings showed no overt phenotype (Fig 3A), and no apparent ectopic expression of hh mRNA and Hh target genes Ara and En was observed in the corresponding wing imaginal discs (Fig 3B,C). Loss of one copy of mtv in a gro1/groE48 mutant background induced an enlarged anterior compartment in the adult wing (Fig 3D) and ectopic expression of hh mRNA, En and Ara in anterior cells (Fig 3E,F). The ectopic expression of En and Ara explains the transformation of anterior wing margin bristles into more posteriorly located sensory organs and the appearance of the ectopic L3. Genetic interactions between gro and mtv mutant alleles, and the similar clonal phenotype suggest that Mtv and Gro might function in the same complex to repress hh expression in anterior cells. This hypothesis was tested using a co-immunoprecipitation assay in S2 cells. Myc-epitope-tagged Mtv was immunoprecipitated with Gro protein and Gro antibody in these cells (Fig 3G). To determine whether the interaction between Gro and Mtv was direct, we carried out a two-hybrid analysis in yeast and a pull-down assay of in vitro-translated Gro and glutathione-S-transferase (GST)-epitope-tagged Mtv (supplementary Fig 2 online). In the yeast assay, Gro did not recognize Mtv, as shown by the lack of expression of the lacZ reporter gene. In the pull-down assay, Mtv was expressed in bacteria as a GST fusion, immobilized on glutathione-Sepharose beads and then tested for their ability to retain radiolabelled Gro protein. GST–Mtv showed little or no binding (supplementary Fig 2 online). Taken together, we can conclude that Gro and Mtv take part in the same protein complex but that they do not bind directly, and we suggest that this complex is important for the repression of hh transcription in anterior cells.
Figure 4.
Anterior hh expression depends on the endogenous activity of the Hh and Notch signalling pathways. Clones of cells mutant for mtv (mtv6; A,B), ptc and mtv (ptcS2mtv6; C–E), gro (Df(3R)Espl22; F,J), ci (ci94; G) or gro and ci (Df(3R)Espl22, ci94; H,I) labelled by the absence (A,C,G), expression (B,D,E) or presence of two copies of the GFP marker (green; F,H–J). Expression of hh-lacZ (light blue, top; white, bottom) was monitored. The endogenous dorsal–ventral boundary is labelled by a dashed line or by Wingless (Wg, red; D) expression. The Hh signalling pathway was blocked by overexpression of a dominant-negative version of Smoothened in all wing cells (Smo-5A) in (A). Using the MARCM technique, the activity of the Hh or Notch signalling pathways was blocked or activated by means of full-length Ptc (B), a dominant-negative form of Mastermind (mam-dn; D) or by an activated form of the Notch receptor (Nintra; E). In (J), CiCell was expressed under the control of MS1096Gal4 control. The MS1096Gal4 domain of expression is depicted by a white line. Gal4 expression levels are higher in the dorsal (d) wing pouch. Ci, Cubitus interruptus; GFP, green fluorescent protein; Gro, Groucho; Hh, Hedgehog; MARCM, mosaic analysis with a repressible cell marker; Mtv, Master of thickveins.
Figure 3.
Gro and Mtv interact in vivo and in vitro. (A,D) Cuticle preparations of gro1/groE48 (A) and mtv6/+;gro1/groE48 (D) adult wings. Note in (D) ectopic veins L2 and L3 (L2*, L3*), and the enlargement of the anterior compartment; compartments a and p are indicated. (B,C,E,F) gro1/groE48 (B,C) and mtv6/+;gro1/groE48 (E,F) wing discs labelled to visualize expression of hh messenger RNA (purple; B,E), Araucan (Ara, blue; C,F) and Engrailed (red; C,F). (G) Co-immunoprecipitation (IP) of Myc-tagged Mtv (Mtv–Myc) by binding to Gro. S2 cells were co-transfected to express Gro and Mtv–Myc or transfected to express Gro or Mtv–Myc alone; empty vector was co-transfected as control in the latter cases. Right panels show 1/30 of Gro and Mtv–Myc proteins in cell lysates; cell lysates were immunoprecipitated with Gro antibody (left). Mtv–Myc was co-immunopreciptitated in significant amounts in cells co-transfected with Gro and Mtv–Myc (lane 1). Mtv–Myc was also co-immunopreciptitated in lesser amount in cells transfected with Mtv–Myc alone (lane 3) as a result of the endogenous Gro present in S2 cells (lanes 11, 12, 15 and 16). In the absence of Gro antibody, Mtv–Myc was not co-immunopreciptitated (data not shown). a, anterior; Gro, Groucho; Hh, hedgehog; Mtv, master of thickveins; p, posterior.
Anterior clones lacking mtv or gro activities induced hh expression only when located close to or abutting the posterior compartment (Figs 1B–D, 4F; Apidianakis et al, 2001). This requirement indicates that ectopic hh expression might depend on the endogenous source of Hh coming from posterior cells. To test this hypothesis, we ubiquitously expressed a dominant-negative version of the transmembrane protein Smoothened (Smo-5A)—an essential component of the Hh signalling pathway. In this background, the activity of the Hh pathway was compromised, as shown by the reduction in Ptc protein levels (supplementary Fig 1 online), and no hh expression was observed in mtv clones (Fig 4A). High levels of Ptc are known to repress the activity of Smo but without affecting the expression of posterior hh (supplementary Fig 1 online). Clones lacking mtv and overexpressing Ptc did not cause ectopic expression of hh either (Fig 4B). Conversely, clones mutant for mtv and constitutively activating the Hh pathway—that is, mutant for ptc—induced hh expression at any distance from the anterior–posterior boundary (Fig 4C). Clones mutant for ptc alone did not have any effect on hh expression (supplementary Fig 1 online). Together, these results indicate that, in the absence of Mtv or Gro activities, anterior hh expression seems to be induced by the Hh signalling pathway.
High levels of Hh signalling leads to the generation of the activator form of Ci (Ciact) and, consequently, to reduced levels of its repressor form Cirep (Aza-Blanc et al, 1997; Ohlmeyer & Kalderon, 1998). Next, we investigated whether anterior hh expression in mtv or gro mutant clones was induced by the activity of Ciact or, alternatively, by the absence of Cirep. Anterior hh expression is independent of Ciact, as clones lacking gro and ci activities still showed the ability to cause ectopic expression of hh at the anterior–posterior boundary (Fig 4H,I), and the levels of hh expression at the anterior–posterior boundary were much higher than in ci single-mutant clones (Fig 4G). Therefore we conclude, that in the absence of mtv or gro activities, anterior hh is expressed only at the anterior–posterior boundary owing to the absence or reduced levels of Cirep. Consistently, when a truncated form of Ci that behaves as a repressor form (CiCell; Méthot & Basler, 1999) was expressed in the dorsal compartment of the wing pouch, hh was not ectopically expressed in gro mutant clones (Fig 4J; note that CiCell also repressed hh expression in posterior cells of the dorsal compartment). We observed that CiCell was able to repress hh in the absence of Gro activity, indicating that Cirep-dependent repression of hh does not require Gro activity.
Anterior hh expression in the absence of Mtv and Gro activities is restricted not only to the anterior–posterior boundary, but also to the region close to or abutting the dorsal–ventral compartment boundary. The Notch signalling pathway is activated at the dorsal–ventral compartment boundary (Diaz-Benjumea & Cohen, 1995; de Celis et al, 1996), where it induces the expression of the long-range morphogen Wingless (Wg). We then investigated whether anterior hh expression depends on the activity of the Notch or Wg signalling pathways. For this purpose, we analysed the effects on hh expression after blocking or activating these pathways. We analysed these effects in clones mutant for both mtv and ptc because in these clones hh expression is induced at any distance from the anterior–posterior boundary. When the Notch signalling pathway was blocked in ptc mtv double-mutant clones by means of expression of a dominant-negative form of the Notch nuclear effector Mastermind (Mam-DN), no hh expression was observed in ptc mtv mutant clones (Fig 4D). When the Notch signalling pathway was constitutively activated by means of a dominant active form of Notch (Struhl & Adachi, 1998), ectopic expression of hh was observed at any distance from the dorsal–ventral boundary (Fig 4E). Expression of Mam-DN or Nintra alone did not have any effect on hh expression (supplementary Fig 1 online). Ectopic expression of Wg in ptc mtv double-mutant clones did not cause any effect in their ability to induce anterior hh expression (data not shown). These results indicate that, in the absence of mtv activity, the Notch and Hh signalling pathways act together to induce hh transcription in anterior cells.
Asymmetrical signalling by Hh from posterior to anterior cells is required for stable compartment subdivision and maintenance of a Dpp-dependent organizer in the centre of the wing primordium. This asymmetry is a consequence of having Hh-expressing and Hh-responding cells in different non-overlapping cell populations corresponding to posterior and anterior compartments, respectively. Two different and independent mechanisms are used in the Drosophila wing to repress hh expression in anterior cells (Fig 1I,J). The first acts mainly in those cells not receiving the Hh signal and is based on the repressor form of Ci (Cirep). We presented evidence that this repression does not require Gro activity. The second acts in those cells receiving the Hh signal and is based on Mtv binding Gro to mediate transcriptional repression of hh. We present evidence that anterior hh expression in the absence of Gro activity does not require Ci. Interestingly, Mtv and Gro seem to be inhibiting a new role of Notch at the dorsal–ventral boundary in driving hh expression in anterior cells. It is interesting to note that in this context the hh gain-of-function allele Moonrat (hhMrt) leads to derepression of hh in anterior cells located at the dorsal–ventral boundary (Fig 2). We speculate that Mtv and Gro might act on a cis-regulatory region of the hh gene affected in the Moonrat allele. Cirep seems to repress hh expression independently of Notch (Fig 4; see also Méthot & Basler, 1999), indicating that Cirep might act on a different cis-regulatory region than Mtv and Gro.
Finally, we would like to point out that many signalling molecules have been shown to restrict their own expression such as Wg or Notch (Rulifson et al, 1996; Herranz et al, 2006) or activity domains such as Hh (Chen & Struhl, 1996). This report shows that Hh is also involved in restricting its expression domain. Hh induces the expression of mtv in nearby anterior cells and Mtv, together with Gro, represses hh expression in anterior cells (Fig 1J).
Methods
Drosophila strains. mtv6, a protein null allelle of mtv, and mtvk00702 (mtv-lacZ in the text; Funakoshi et al, 2001); hhMrt (Felsenfeld & Kennison, 1995); Df(2R)en[E] deletes both en and invected (Gustavson et al, 1996); c765-Gal4 and UAS-Smo-5A (Collins & Cohen, 2005); UAS-Nintra (Struhl & Adachi, 1998); UAS-mam-DN (Helms et al, 1999); ptcs2, hhP30 (hh-lacZ in the text), ci-lacZ, Df(3R)Espl22, gro1 and groE48 (Flybase); ci94 and UAS-CiCell (Méthot & Basler, 1999); ap-Gal4 and MS1096-Gal4 (Milan et al, 1998).
Antibodies. Rabbit anti-Ara (Gomez-Skarmeta & Modolell, 1996), rabbit anti-Hh (Capdevila & Guerrero, 1994), rat anti-Ci (Motzny & Holmgren, 1995), mouse anti-Mtv (Senti et al, 2000), mouse anti-Gro (Apidianakis et al, 2001); rabbit anti-Myc (Santa Cruz Biotechnology Inc., Antibodies, Santa Cruz, CA, USA) and rabbit anti-β-Gal (Cappel). Mouse anti-En (4D9), mouse anti-Wg (4D4), mouse anti-Ptc (Apa 1), mouse anti-Dl (C594.9B) and mouse anti-β-Gal (40-1A) are described in the Developmental Studies Hybridoma Bank (University of Iowa, Iowa, USA). In situ hybridization was carried out as described by Milan et al (1996).
Co-immunoprecipitation of Gro and Mtv. S2 cells were transfected two times each with 1 μg of pMT-Gal4-VP16 and UAS-Myc-Mtv and UAS-Gro or empty vector using 5 μl of CellFectin (Invitrogen, Carlsbad, CA, USA) per well. Cells were recovered for 6 h after transfection, induced for 2 days with 0.7 mM CuSO4 and lysed in 500 μl of 5 mM Tris, 150 mM NaCl and 1% Triton X-100 (pH 8). A portion (1/30th) was analysed on SDS–polyacrylamide gel electrophoresis (SDS–PAGE). Medium fractions (450 ml) were each immunoprecipitated with Gro antibody overnight at 4°C. A 50 μl portion of protein G slurry was added for 30 min at 4°C. The beads were washed three times with PBS and 0.1% Triton X-100, and then boiled in 50 μl of 2 × SDS–PAGE loading buffer and one-third was loaded on SDS–PAGE. All buffers were supplemented with protease inhibitors (Boehringer Ingelheim Gmbh, Ingelheim, Germany). Blots were probed with mouse anti-Myc and mouse anti-Gro.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/v8/n8/extref/7401003-s1.pdf, http://www.nature.com/embor/journal/v8/n8/extref/7401003-s2.pdf, http://www.nature.com/embor/journal/v8/n8/extref/7401003-s3.pdf).
Supplementary Material
supplementary Figure Legends and Information
supplementary Figure 1
supplementary Figure 2
Acknowledgments
We thank K. Basler, S. Campuzano, S. Cohen, B. Dickson, I. Guerrero, G. Jiménez, Z. Paroush, T. Tabata, J. Treisman and the Bloomington Stock Center (Indiana, USA) and the Developmental Studies Hybridoma Bank (University of Iowa, Iowa, USA) for flies and reagents; Z. Paroush and G. Jiménez for their invaluable advice in the yeast two-hybrid and GST pull-down assays; and I. Becam, J. Casanova, S. Cohen, I. Guerrero, H. Herranz and T. Yates for comments on the manuscript. F.B. was funded by a postdoctoral fellowship from the Ministerio de Educación y Ciencia, Spain; M.M.'s laboratory by a grant from the Dirección General de Investigación Científica y Técnica, (BFU2004-00167/BMC), a grant from the Generalitat de Catalunya (2005 SGR 00118) and intramural funds; and C.D.'s laboratory by the Program for the reinforcement of the Research Staff (PENED) programme of the General Secretariat for Research and Technology, Greece, and the Institute of Molecular Biology and Biotechnology (IMBB) intramural funds.
References
- Apidianakis Y, Grbavec D, Stifani S, Delidakis C (2001) Groucho mediates a Ci-independent mechanism of hedgehog repression in the anterior wing pouch. Development 128: 4361–4370 [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P, Ramírez-Weber F-A, Laget M-P, Schwartz C, Kornberg TB (1997) Proteolysis that is inhibited by Hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell 89: 1043–1053 [DOI] [PubMed] [Google Scholar]
- Basler K, Struhl G (1994) Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature 368: 208–214 [DOI] [PubMed] [Google Scholar]
- Capdevila J, Guerrero I (1994) Targeted expression of the signalling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J 13: 4459–4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Struhl G (1996) Dual roles for patched in sequestering and transducing Hedgehog. Cell 87: 553–563 [DOI] [PubMed] [Google Scholar]
- Collins RT, Cohen SM (2005) A genetic screen in Drosophila for identifying novel components of the hedgehog signaling pathway. Genetics 170: 173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick FH, Lawrence PA (1975) Compartments and polyclones in insect development. Science 189: 340–347 [DOI] [PubMed] [Google Scholar]
- de Celis JF, Ruiz-Gomez M (1995) Groucho ahd hedgehog regulate engrailed expression in the anterior compartment of the Drosophila wing. Development 121: 3467–3476 [DOI] [PubMed] [Google Scholar]
- de Celis JF, Garcia-Bellido A, Bray SJ (1996) Activation and function of Notch at the dorsal–ventral boundary of the wing imaginal disc. Development 122: 359–369 [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea FJ, Cohen SM (1995) Serrate signals through Notch to establish a wingless-dependent organizer at the dorsal–ventral compartment boundary of the Drosophila wing. Development 121: 4215–4225 [DOI] [PubMed] [Google Scholar]
- Felsenfeld AL, Kennison JA (1995) Positional signaling by hedgehog in Drosophila imaginal disc development. Development 121: 1–10 [DOI] [PubMed] [Google Scholar]
- Freeman M, Gurdon JB (2002) Regulatory principles of developmental signaling. Annu Rev Cell Dev Biol 18: 515–539 [DOI] [PubMed] [Google Scholar]
- Funakoshi Y, Minami M, Tabata T (2001) mtv shapes the activity gradient of the Dpp morphogen through regulation of thickveins. Development 128: 67–74 [DOI] [PubMed] [Google Scholar]
- García-Bellido A, Ripoll P, Morata G (1973) Developmental compartmentalisation of the wing disk of Drosophila. Nat New Biol 245: 251–253 [DOI] [PubMed] [Google Scholar]
- Gomez-Skarmeta JL, Modolell J (1996) araucan and caupolican provide a link between compartment subdivisions and patterning of sensory organs and veins in the Drosophila wing. Genes Dev 10: 2935–2945 [DOI] [PubMed] [Google Scholar]
- Gustavson E, Goldsborough AS, Ali Z, Kornberg TB (1996) The Drosophila engrailed and invected genes: partners in regulation, expression and function. Genetics 142: 893–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms W, Lee H, Ammerman M, Parks AL, Muskavitch MA, Yedvobnick B (1999) Engineered truncations in the Drosophila mastermind protein disrupt Notch pathway function. Dev Biol 215: 358–374 [DOI] [PubMed] [Google Scholar]
- Herranz H, Stamataki E, Feiguin F, Milan M (2006) Self-refinement of Notch activity through the transmembrane protein Crumbs: modulation of γ-Secretase activity. EMBO Rep 7: 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo A (1994) Three distinct roles for the engrailed gene in Drosophila wing development. Curr Biol 4: 1087–1098 [DOI] [PubMed] [Google Scholar]
- Méthot N, Basler K (1999) Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell 96: 819–831 [DOI] [PubMed] [Google Scholar]
- Milan M, Campuzano S, Garcia-Bellído A (1996) Cell-cycling and patterned cell proliferation in the wing primordium of Drosophila. Proc Natl Acad Sci USA 93: 640–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan M, Diaz-Benjumea FJ, Cohen SM (1998) Beadex encodes an LMO protein that regulates Apterous LIM-homeodomain activity in Drosophila wing development: a model for LMO oncogene function. Genes Dev 12: 2912–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzny CK, Holmgren RA (1995) The Drosophila cubitus interruptus protein and its role in wingless and hedgehog signal transduction pathways. Mech Dev 52: 137–150 [DOI] [PubMed] [Google Scholar]
- Ohlmeyer JT, Kalderon D (1998) Hedgehog stimulates maturation of Cubitus interruptus into a labile transcriptional activator. Nature 396: 749–753 [DOI] [PubMed] [Google Scholar]
- Rulifson EJ, Micchelli CA, Axelrod JD, Perrimon N, Blair SS (1996) Wingless refines its own expression domain on the Drosophila wing margin. Nature 384: 72–74 [DOI] [PubMed] [Google Scholar]
- Senti K, Keleman K, Eisenhaber F, Dickson BJ (2000) Brakeless is required for lamina targeting of R1–R6 axons in the Drosophila visual system. Development 127: 2291–2301 [DOI] [PubMed] [Google Scholar]
- Struhl G, Adachi A (1998) Nuclear access and action of Notch in vivo. Cell 93: 649–660 [DOI] [PubMed] [Google Scholar]
- Tabata T, Kornberg T (1994) Hedgehog is a signalling protein with a key role in patterning Drosophila imaginal discs. Cell 76: 89–102 [DOI] [PubMed] [Google Scholar]
- Tabata T, Schwartz C, Gustavson E, Ali Z, Kornberg TB (1995) Creating a Drosophila wing de novo: the role of engrailed and the compartment border hypothesis. Development 121: 3359–3369 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Figure Legends and Information
supplementary Figure 1
supplementary Figure 2